Transmembrane Proteins
By Christian Lautenschlager, PhD and Ryan Hamnett, PhD
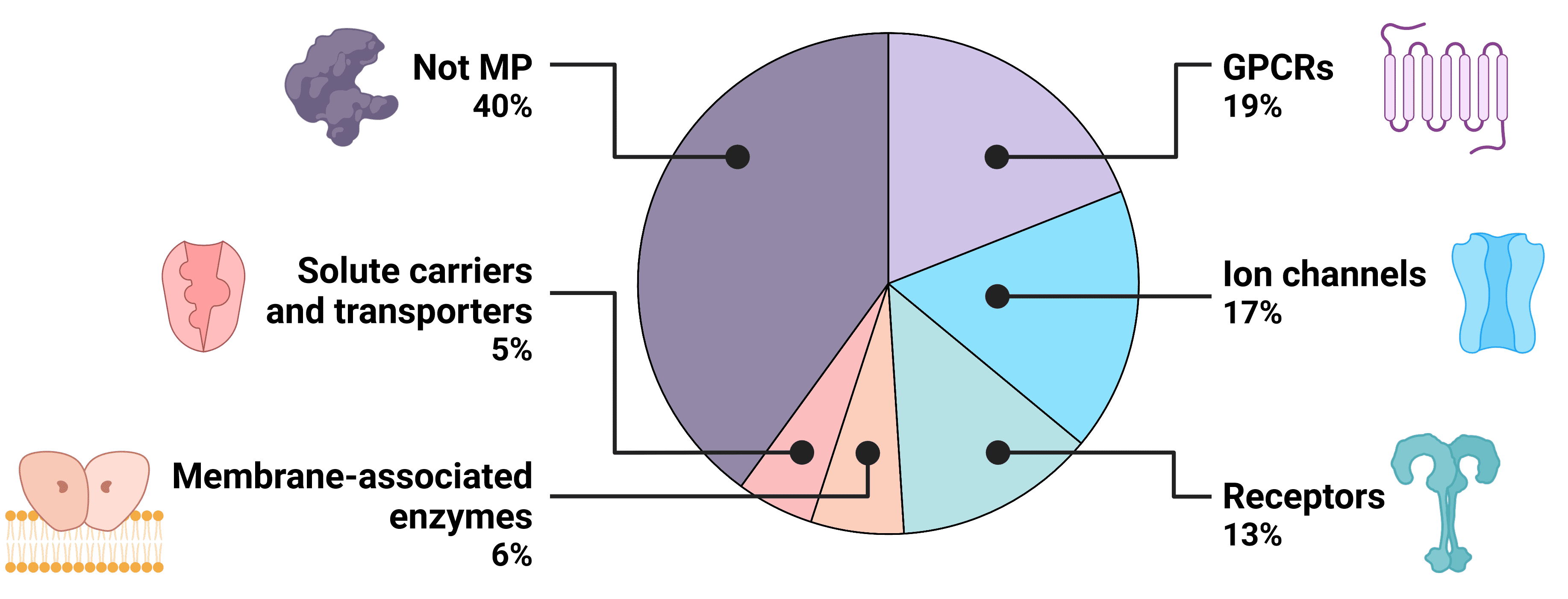
Transmembrane proteins (TMPs) span the lipid bilayer of living cells, acting as bridges across which the external environment can regulate cellular activity. Transmembrane proteins are involved in diverse cellular functions, including material transport, signal transmission, and cell-cell recognition, making them prominent targets for drug development to modulate cellular processes or target specific cell types. Though transmembrane proteins make up just 23% of the human proteome, over 60% of known drugs now target transmembrane proteins1,2, including BCMA, GPRC5D, and CCR8, for the treatment of conditions such as cancer, cardiovascular disorders, neurological conditions and autoimmune disorders.

Figure 1: The druggable human proteome.
However, the isolation and study of transmembrane proteins, especially multi-pass transmembrane proteins with multiple hydrophobic transmembrane regions, can be technically demanding. In contrast to single-pass transmembrane proteins, which can typically be studied via their extracellular domain (ECD), full-length multi-pass transmembrane proteins must be used. Hydrophobic regions make them susceptible to in vitro aggregation, while their three-dimensional structure and conformation, essential to maintain for functional assays and structural biology, are sensitive to environmental conditions.
Through technical innovation within this complex landscape, we provide a suite of research tools to enable you to study transmembrane proteins and generate unprecedented insights into transmembrane protein structure, function, and interactions.
Table of Contents
Synthetic Nanodiscs
Nanodiscs are nanoscale structures (10-50 nm) comprising transmembrane proteins within a phospholipid bilayer, held by a stabilising belt. First conceived by the laboratory of Professor Stephen G. Sligar in 20023, nanodiscs offer a stable and biologically relevant environment to enable the isolation of TMPs in their native conformation.
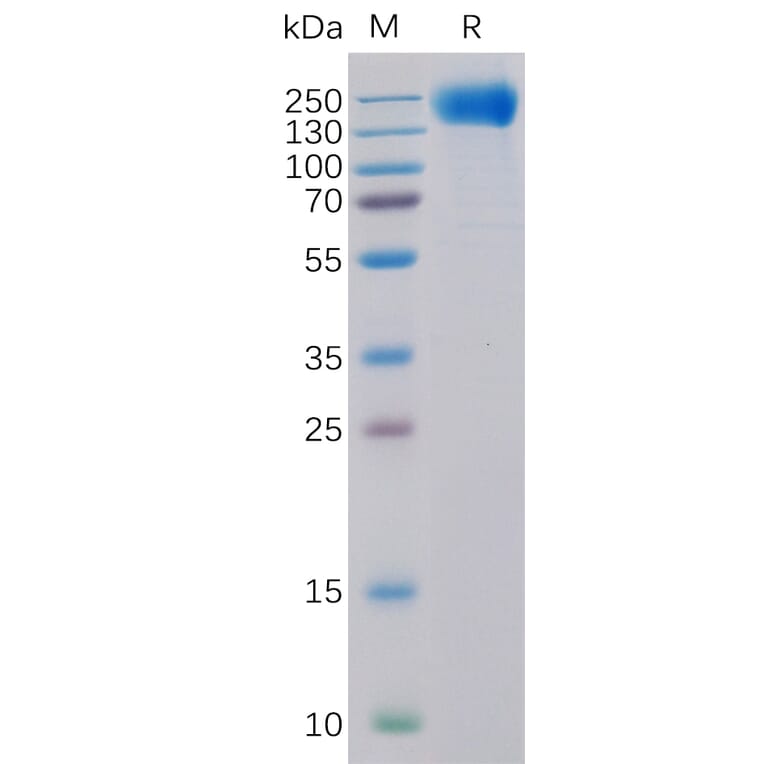
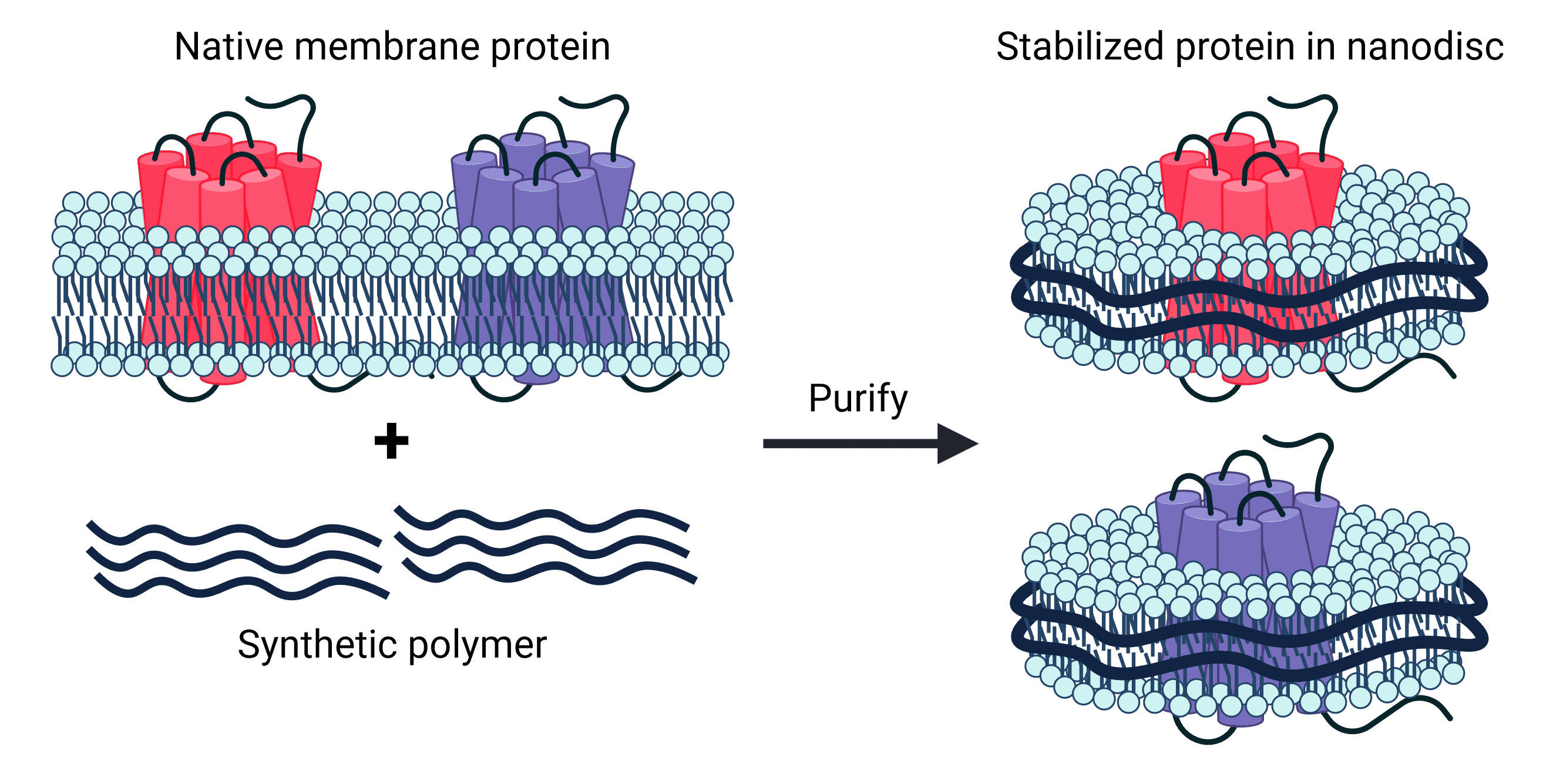
Synthetic nanodiscs, one of the two types of nanodisc, are composed of native cell membrane phospholipids encircled by belts made from a variety of synthetic polymers, such as styrene-maleic acid co-polymers (SMAs) or Diisobutylene-maleic acid (DIBMA), offering great control and versatility in application4,5. These synthetic belts have been engineered for stability and solubility, meaning no detergents are required in the isolation of transmembrane proteins held within the nanodiscs. Furthermore, nanodisc proteins are produced in mammalian cell expression systems, which ensure proper folding and post-translational modifications (PTMs) of proteins. This is particularly important for complex multi-pass transmembrane proteins, which often require specific chaperones and co-factors for assembly.
Figure 2: Synthetic nanodiscs containing full-length TMPs in a phospholipid bilayer are generated from native cell membranes.
Advantages of Nanodiscs:
- Detergent-free purification process.
- They provide a stable environment for TMPs in vitro - preserving their natural conformation and activity.
- They are versatile and can be employed across numerous applications (see below).
- Nanodiscs show promise in vaccine preparation and antibody production.
Nanodisc Applications:
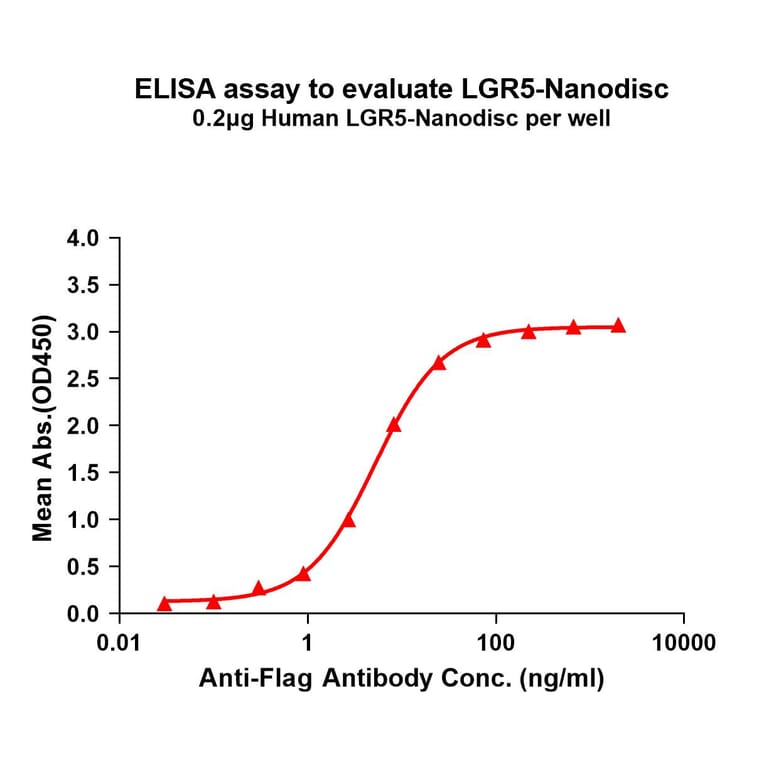
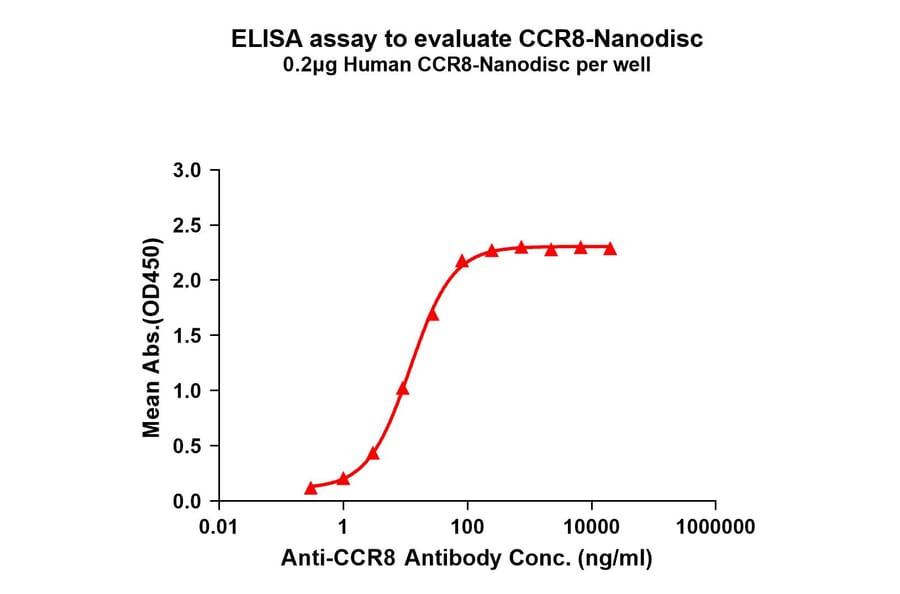
- Enzyme-Linked Immunosorbent Assay (ELISA)
- Nanodiscs facilitate immobilisation on ELISA plates for detection.
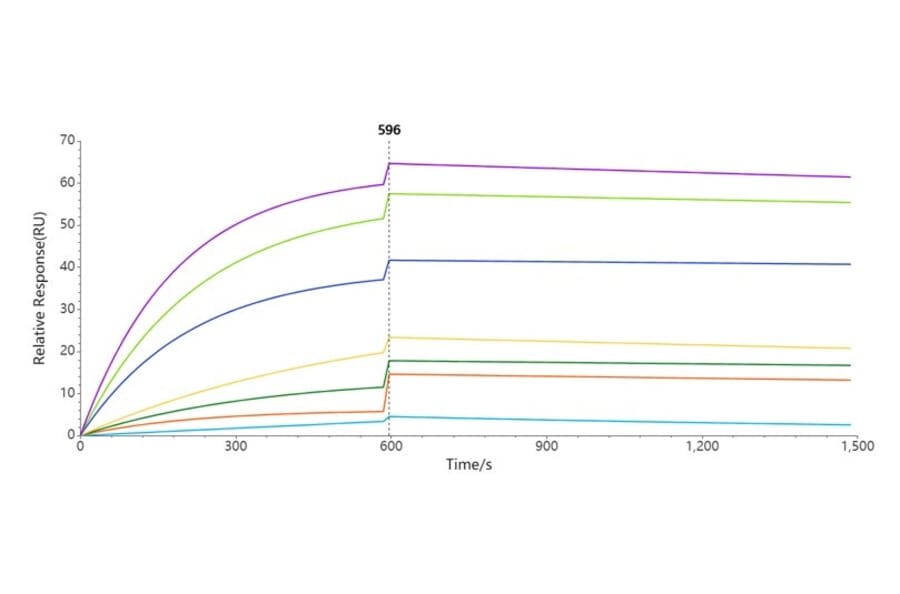
- Surface Plasmon Resonance (SPR) Affinity Analysis
- Through immobilisation of transmembrane proteins on SPR chips.
- Chimeric Antigen Receptor (CAR) Expression Analysis
- Incorporation of CARs into nanodiscs for downstream characterization.
- Protein Crystallisation
- Enables TMPs to be stabilised and subjected to crystallisation conditions for structural analysis.
- Phage Display Screening
- Transmembrane proteins within nanodiscs can be targeted in phage display libraries.
- Toxicology
- To study the effects of drugs or environmental factors on transmembrane proteins.
- Vaccine Development
- Enhance stability and immunogenicity of transmembrane protein antigens.
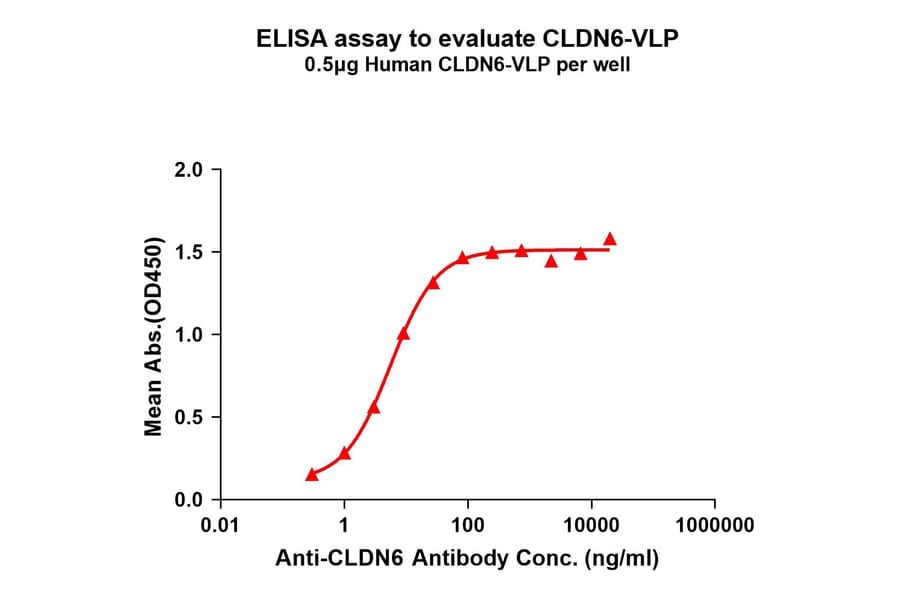
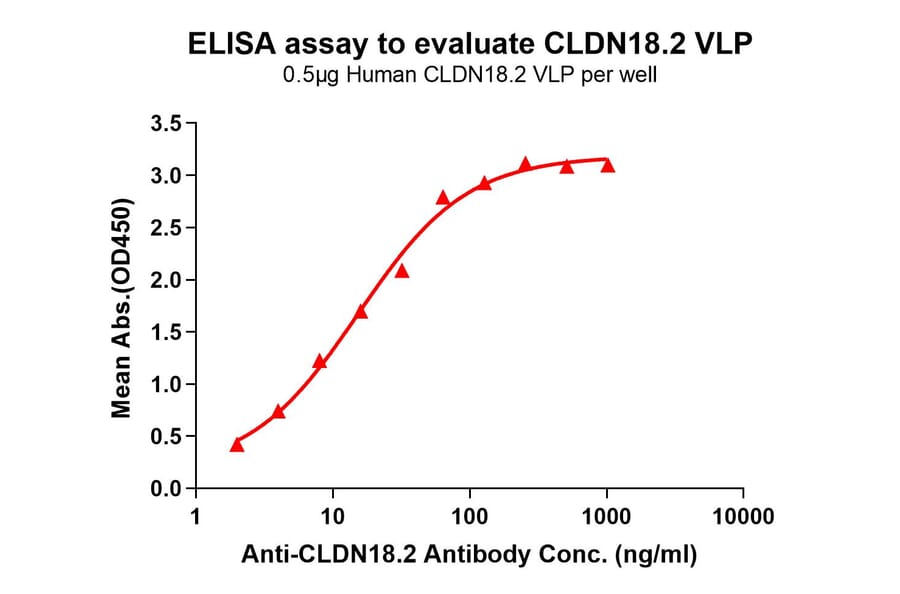
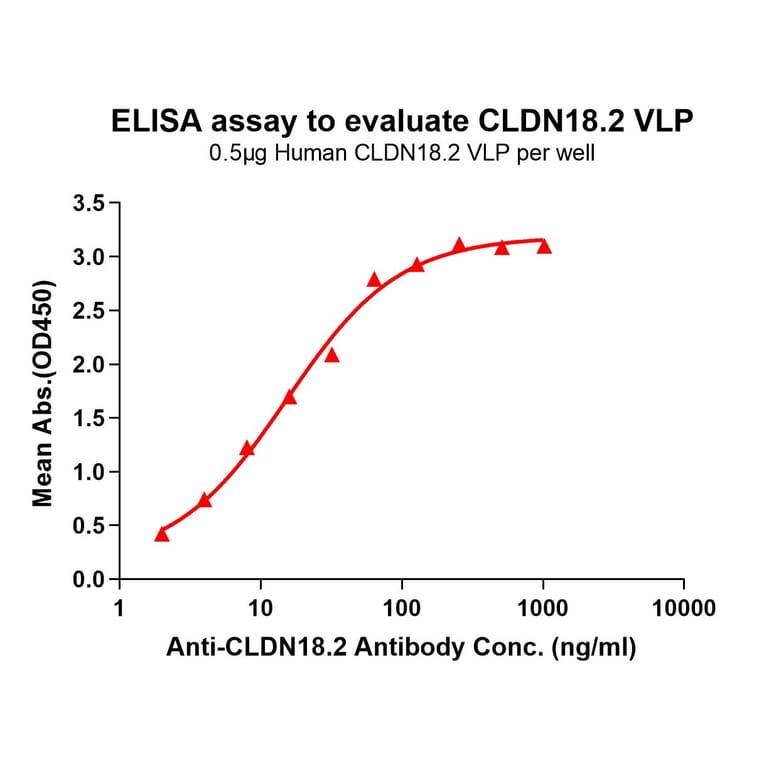
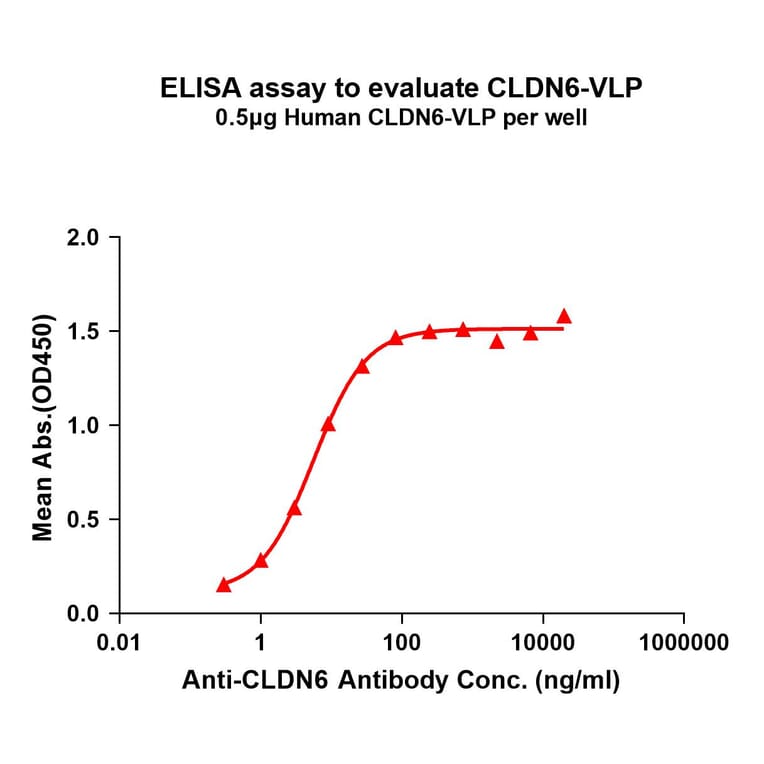
Virus-like Particles (VLPs)
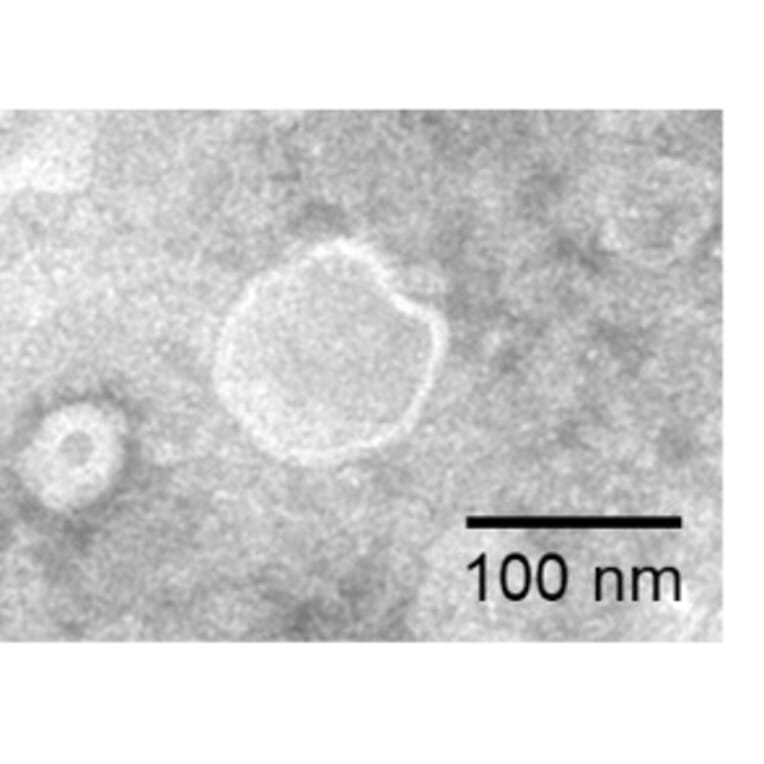
Virus-like particles (VLPs) are non-infectious structures that mimic the shape of viruses but lack a viral genome. During VLP production in cells or cell-free systems, VLPs self-assemble into 100-150 nm protein particles, and incorporate transmembrane proteins into a VLP-MP complex, resulting in presentation of the protein on the external surface of the VLP.
Figure 5: VLP-TMP complexes are generated containing full-length transmembrane proteins following self-assembly.
Advantages of VLPs:
- Proteins are highly expressed and purified without the need for detergents.
- VLP-TMPs deliver high quality immunogens for antibody development and screening.
- The transmembrane protein is maintained in its native structure and orientation.
- Engineered to have only specific transmembrane protein molecules on VLP surface.
- High immunogenicity for vaccine production.
VLP Applications:
- Vaccine Development
- VLPs can be based on a variety of viruses, including hepatitis B virus, human papillomavirus and influenza virus.
- Surface Plasmon Resonance (SPR) Affinity Analysis
- Through immobilisation of transmembrane proteins on SPR chips.
- Antibody Production
- VLPs are useful for displaying transmembrane proteins at high density.
- Ligand Binding Experiments
- Used to analyse kinetics of receptor-ligand binding.
- Drug Discovery
- The isolated VLP-TMP may be used to screen large compound libraries in a high throughput screening format.
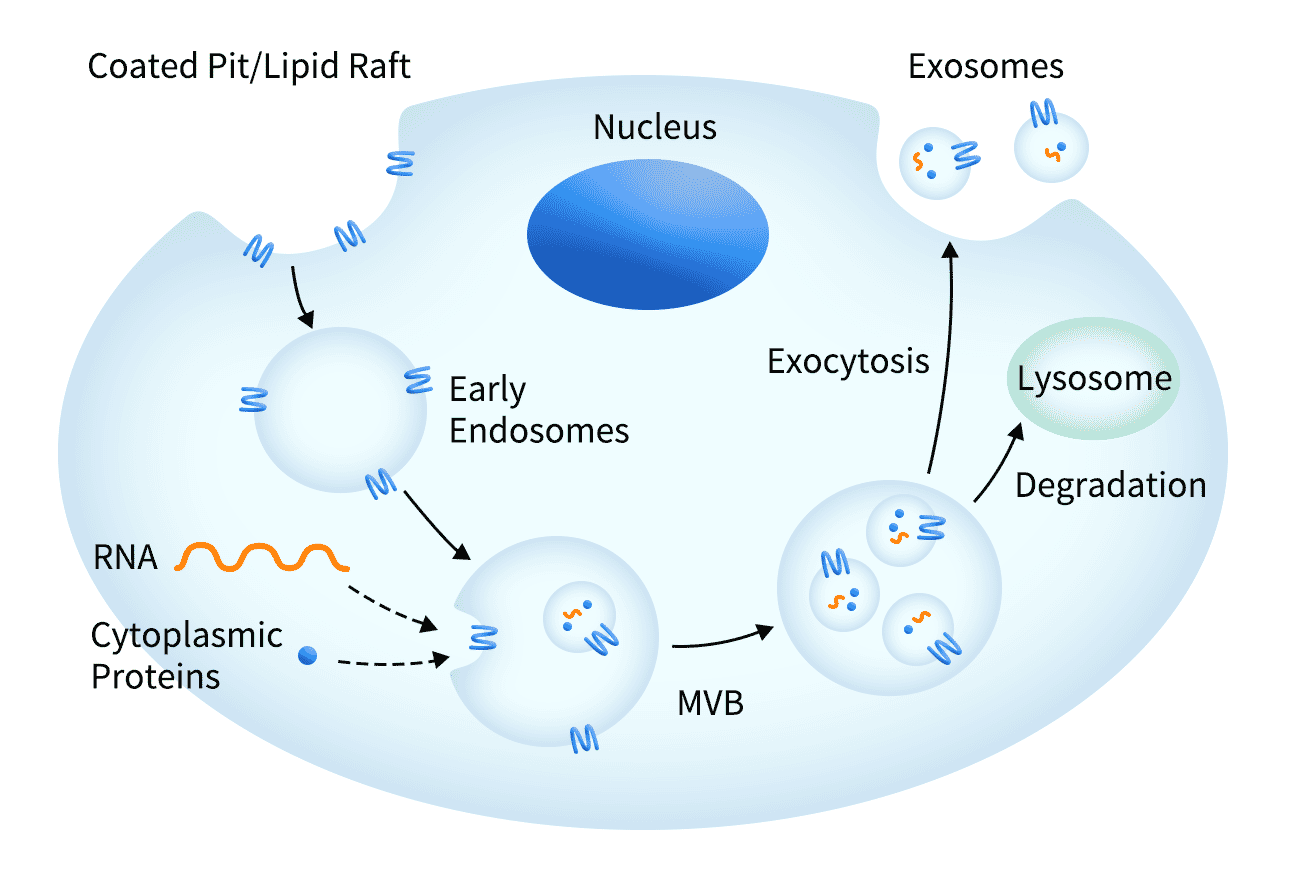
Exosomes
Exosomes are small (30-150 nm) membrane-bound vesicles secreted by the cell, which mediate cell-cell communication and play an important role in the immune response. Each vesicle contains a variety of molecules including proteins, RNA and DNA.
Exosomes are useful for supporting transmembrane protein molecules by providing an optimal environment to maintain receptor solubility. The transmembrane proteins are overexpressed, localised to the host cell membrane, and subsequently secreted within exosomes, which are purified for downstream applications. As a result of the exosome being secreted, there is no need to perform harsh membrane extraction techniques which is sometimes necessary with traditional techniques.
Figure 8: Exosomes are naturally formed and secreted by cells, containing transmembrane proteins in the cell membrane as well as other biological molecules.
Advantages of Exosomes:
- High expression levels of the transmembrane protein.
- Detergent-free purification process.
- Mimics native structure and orientation of the natural transmembrane protein.
- Generates a strong immune response when used as an immunogen.
Exosome Applications:
- Antibody Development
- Due to high expression and concentration of the transmembrane protein in the exosome.
- Drug Discovery
- Screening compound libraries in an in vitro, high content screening assay.
- Characterising target TMPs
- Binding kinetics and biological function can be assayed when the MP is stably localised within exosomes.
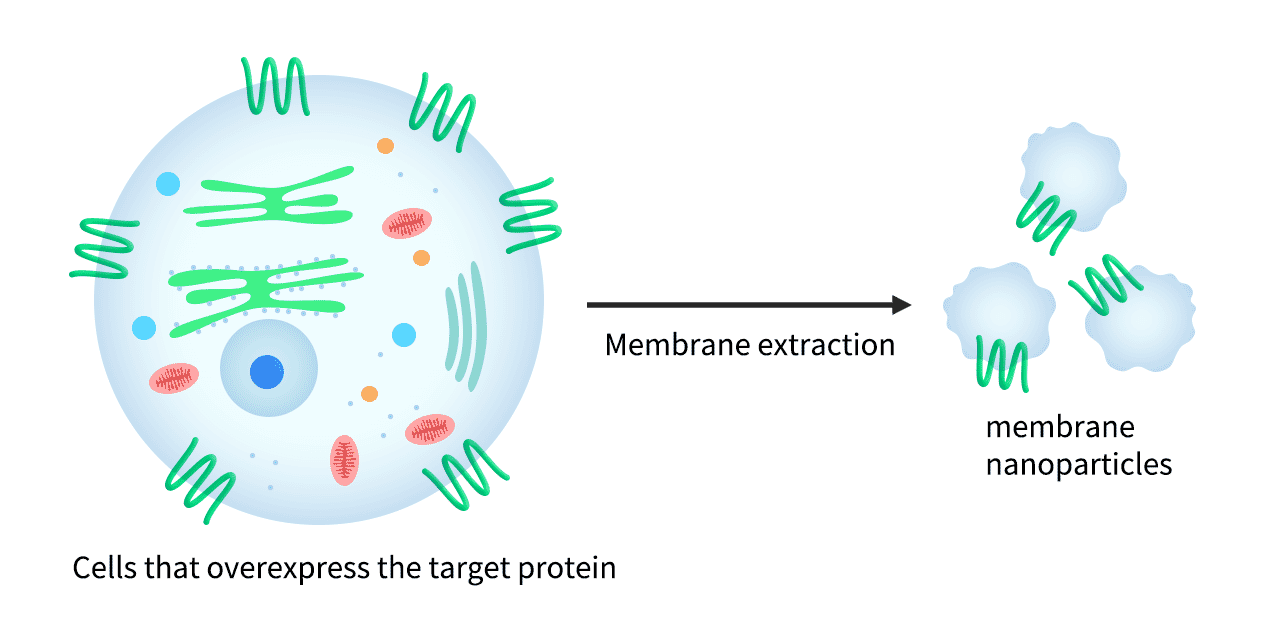
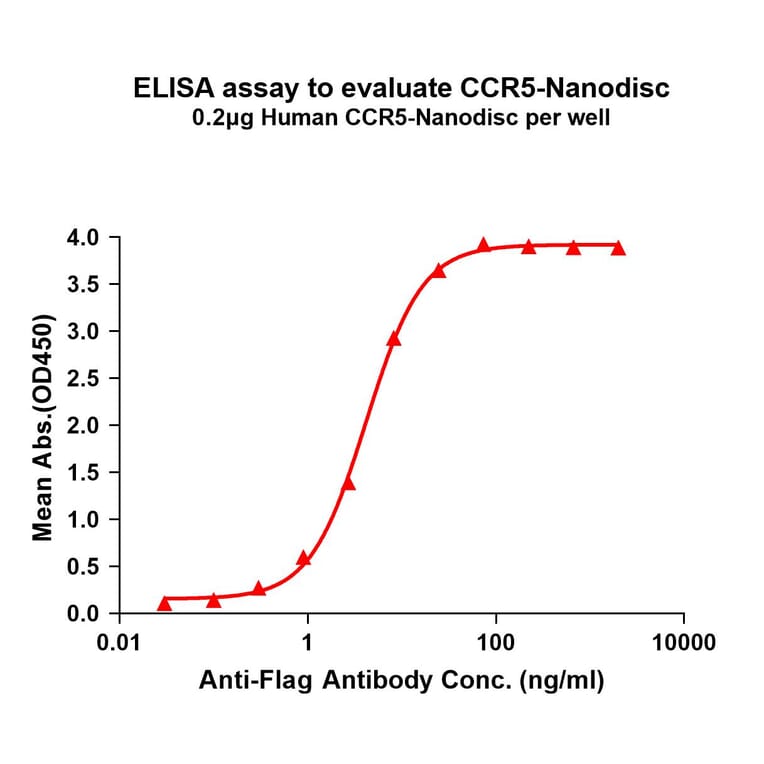
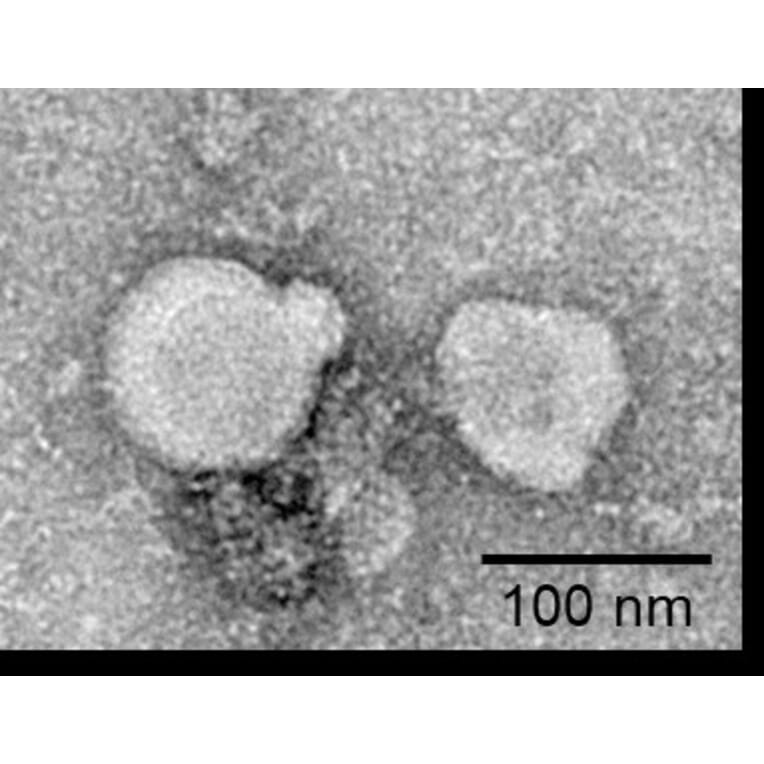
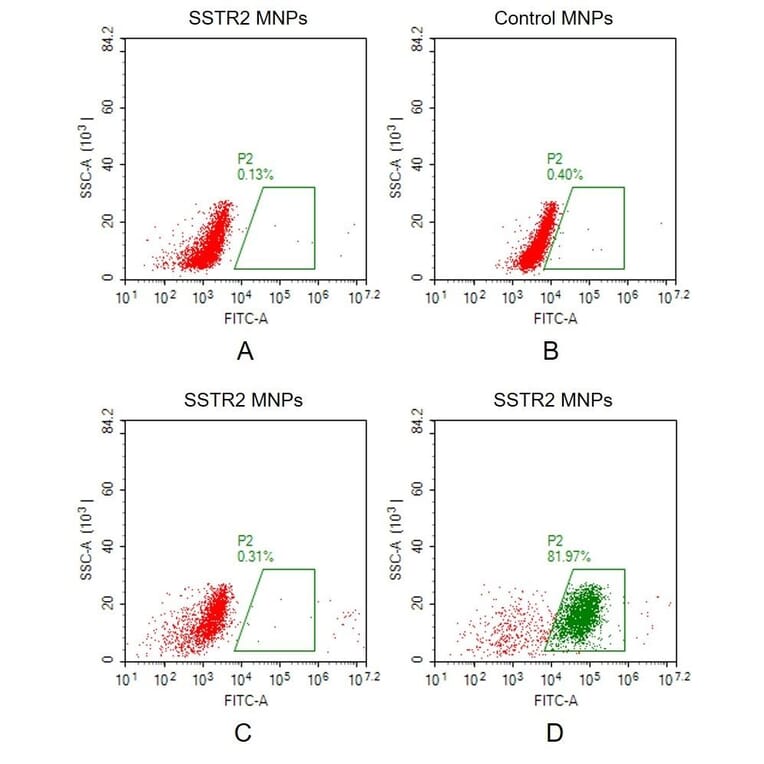
Membrane Nanoparticles (MNPs)
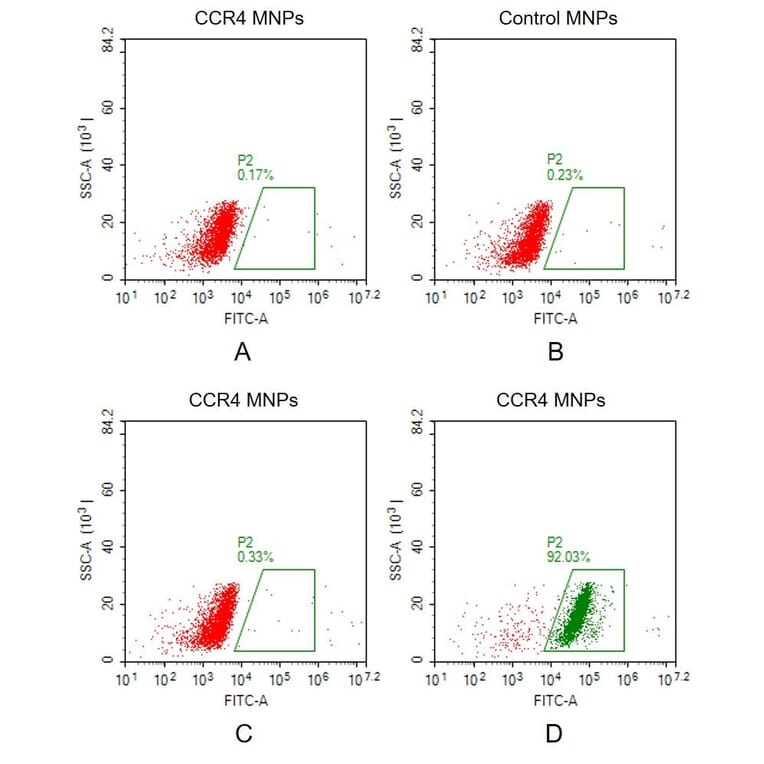
Membrane nanoparticles (MNPs) comprise a synthetic nanoparticle coated with a layer of natural cell membrane. Membrane nanoparticles are extremely useful for isolating transmembrane proteins because of their small size (10-100 nm) and because the lipid bilayer maintains MP stability and solubility.
To form MNP-TMP complexes, the transmembrane protein is expressed within HEK293 cells and displayed on the cell surface, before proprietary extraction processes are used to isolate the membrane from the host cells and produce the target protein-containing membrane nanoparticles.
Figure 9: Overexpressed TMPs within the cell membrane are extracted and packaged as membrane nanoparticles.
Advantages of Membrane Nanoparticles (MNPs):
- Expression within HEK293 cells supports PTMs, and maintains native protein conformation and activity.
- Generate strong immune response when used as immunogens.
- Detergent-free purification process.
- Soluble in aqueous solutions for routine biochemical analysis.
Membrane Nanoparticles (MNPs) Applications:
- ELISA
- SPR Affinity Analysis
- Phage Display Screening
- Antibody Generation
- CAR T Cell Screening
Extracellular Domain (ECD) Proteins
Extracellular domain (ECD) proteins refers to the region of an transmembrane protein located on the outside of the cell. Many drugs against transmembrane proteins target the ECD, given their exposure and role in interacting with other cells and signalling molecules. We provide over 1,000 purified extracellular domain proteins, made using the HEK293 mammalian cell expression system. To ensure the highest quality standards, we assess purity, antibody-drug interaction, and stability testing.
Advantages of Extracellular Domain (ECD) Proteins:
- Manufacture using HEK293 mammalian cell expression system ensures close-to-native structures and PTMs.
- Rigorous purity and stability testing.
Extracellular Domain (ECD) Proteins Applications:
- Antibody Development
- Extracellular domain proteins can act as immunogens.
- Drug Discovery
- Screening compound libraries in in vitro, high content screening assays.
- Characterisation of Transmembrane Proteins
- Assays for binding kinetics and biological function of the extracellular domain proteins.
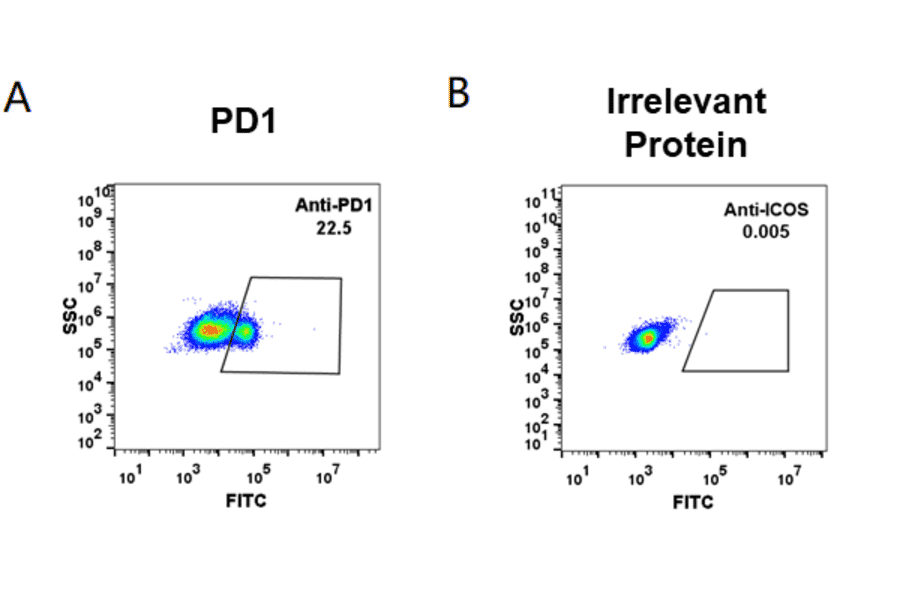
Biosimilar Antibodies
Biosimilar antibodies are designed to mimic existing, FDA approved therapeutic antibodies, but are manufactured for research use only (RUO). They have a wide range of applications in biological research, including as standards to compare new lead candidates against for binding affinity, association and dissociation. In the study of TMPs, biosimilar antibodies can be used for functional characterisation of a target TMP.
Advantages of Biosimilar Antibodies
- Wide range of applications in biological research.
- Providing non-therapeutic biosimilar antibodies for RUO avoids the need to source therapeutic products, which can be expensive and only available in large quantities.
Biosimilar Antibody Applications:
- Immunohistochemistry
- Detecting the expression, localisation and distribution of transmembrane proteins.
- Functional Studies, Drug Target Validation
- Biosimilar antibodies can target functional domains of TMPs, allowing studies on how blocking specific domains modulates TMP function, which may validate its potential as a drug target.
- Immunoprecipitation
- Transmembrane proteins and any associated proteins can be isolated using biosimilar antibodies.
- Surface Plasmon Resonance (SPR)
- To study the binding kinetics between the antibody and the transmembrane protein.
Popular Multipass Transmembrane Proteins
References
- Overington, J. P., Al-Lazikani, B. & Hopkins, A. L. How many drug targets are there? Nat. Rev. Drug Discov. 5, 993–996 (2006).
- Tiefenauer, L. & Demarche, S. Challenges in the Development of Functional Assays of Membrane Proteins. Materials 5, 2205–2242 (2012).
- Bayburt, T. H., Grinkova, Y. V. & Sligar, S. G. Self-Assembly of Discoidal Phospholipid Bilayer Nanoparticles with Membrane Scaffold Proteins. Nano Lett. 2, 853–856 (2002).
- Dörr, J. M. et al. The styrene–maleic acid copolymer: a versatile tool in membrane research. Eur. Biophys. J. 45, 3–21 (2016).
- Chen, A., Majdinasab, E. J., Fiori, M. C., Liang, H. & Altenberg, G. A. Polymer-Encased Nanodiscs and Polymer Nanodiscs: New Platforms for Membrane Protein Research and Applications. Front. Bioeng. Biotechnol. 8, (2020).


![ELISA - Anti-PD-L1 Humanized Antibody [Atezolizumab Biosimilar] - Azide free (A318947) - Antibodies.com](https://cdn.antibodies.com/image/catalog/318/A318947_1.png?profile=product_image)
![Recombinant Anti-CD20 Chimeric Antibody [Rituximab Biosimilar] (A318931) - Antibodies.com](https://cdn.antibodies.com/image/catalog/318/A318931_1.png?profile=product_top)
![Recombinant Anti-Integrin alpha 4 / CD49D Humanized Antibody [Natalizumab Biosimilar] (A318888) - Antibodies.com](https://cdn.antibodies.com/image/catalog/318/A318888_3.jpg?profile=product_top)