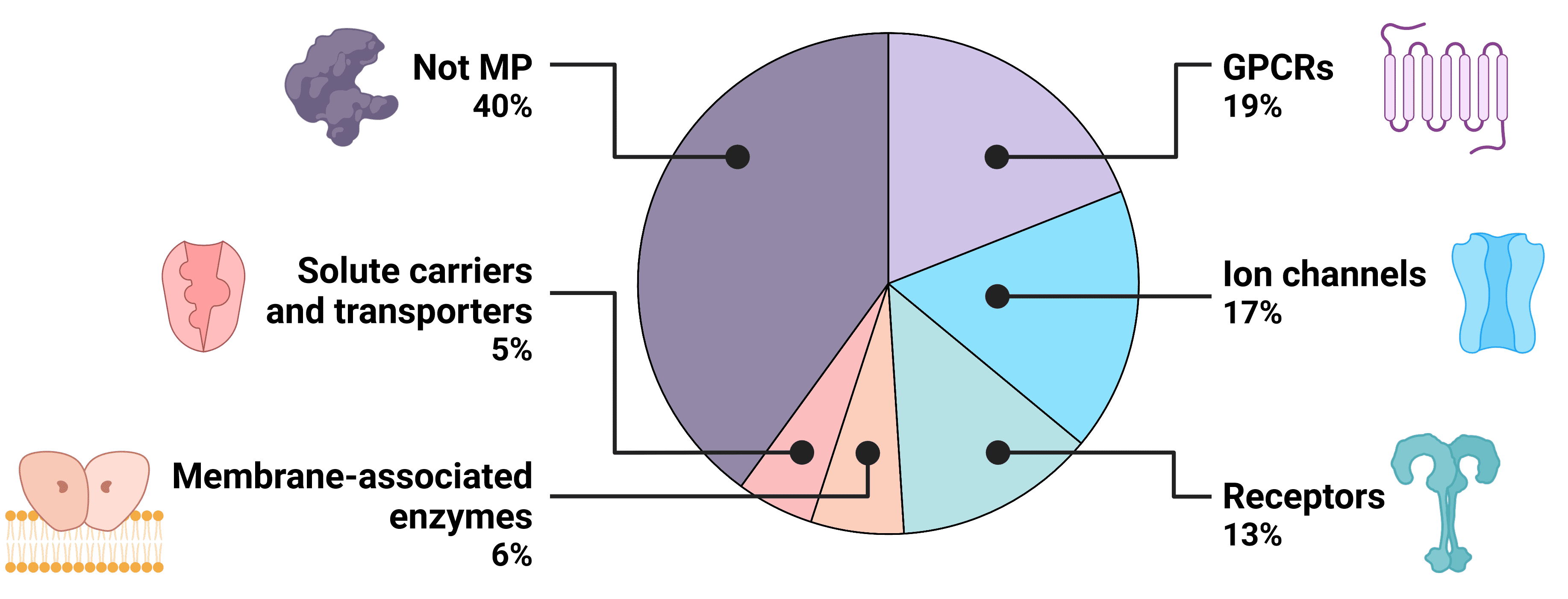
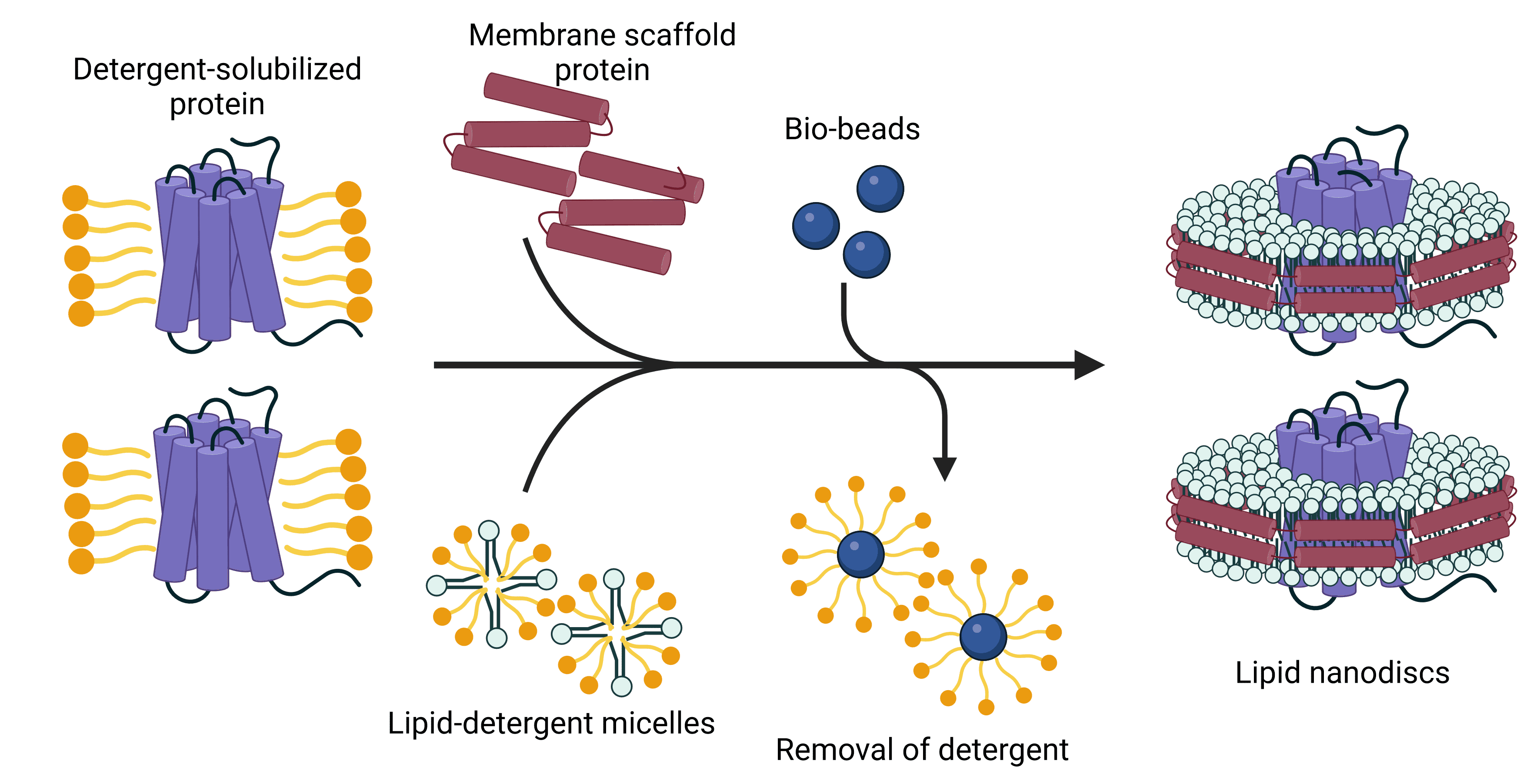
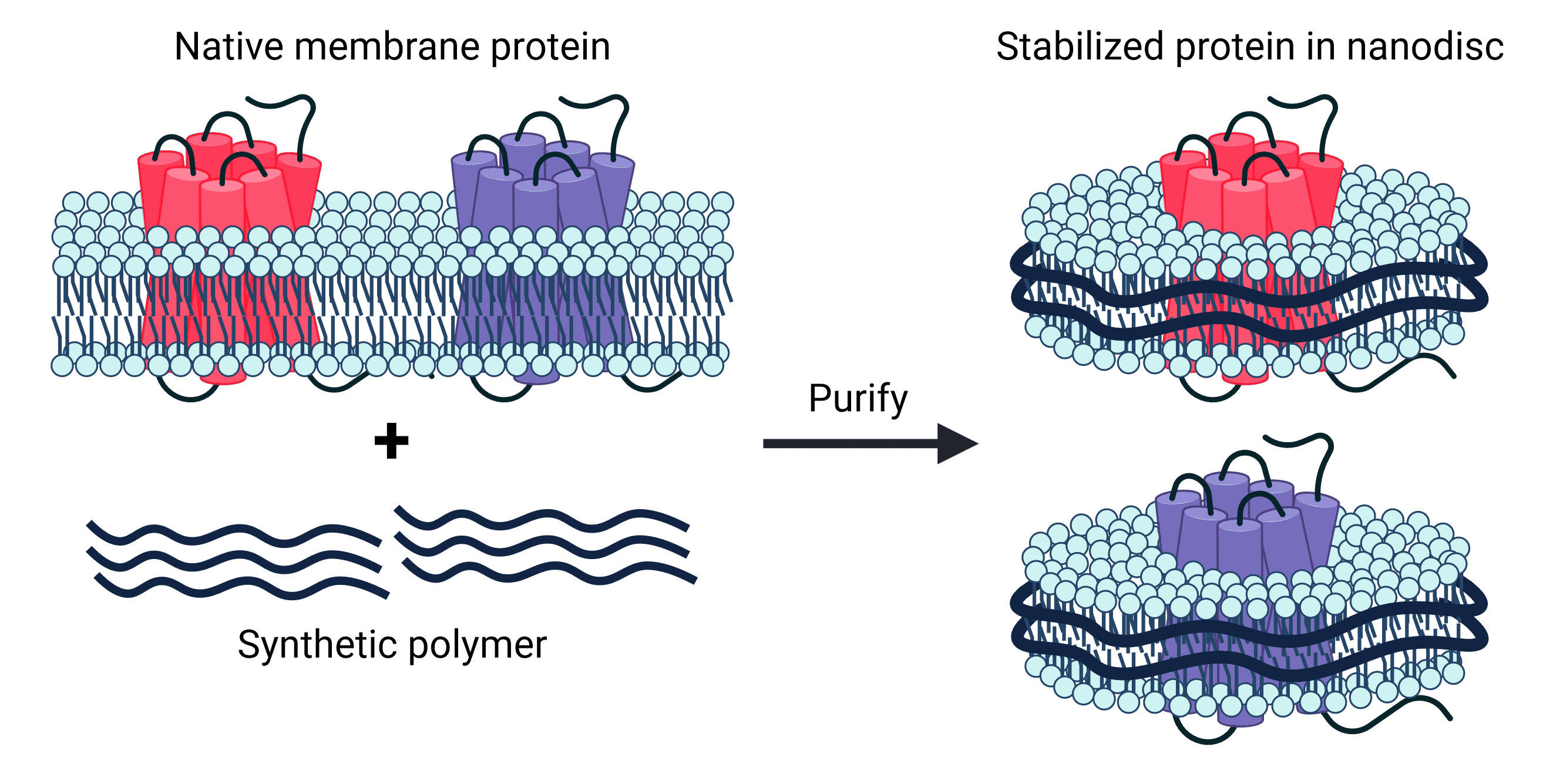
Research Applications
Nanodiscs are an ideal platform for studying the structure and function of membrane proteins with a wide range of biochemical and biophysical techniques.4 Different sizes of proteins or protein complexes can be accommodated by changing the size of the nanodisc with altered lipid to polymer ratios. This control over their composition and size results in a high degree of homogeneity, which is particularly useful for structural studies.
Structural Studies
Structural studies of membrane proteins benefit from several aspects of nanodiscs. A structural investigation ideally reveals not only the physical conformation of the target protein, but also its topology within a lipid bilayer and how it interacts with the lipids around it.4 Nanodiscs are well-suited to this, containing proteins within their native membrane environment. High concentrations of protein are often required for structural techniques such as nuclear magnetic resonance (NMR), but this is challenging when working with membrane proteins due to their propensity to aggregate. The protection of hydrophobic domains afforded by the lipid bilayer of nanodiscs prevents this, meaning high concentrations can be achieved without aggregation or high viscosity.
As a result of these traits, nanodiscs are applicable to structural techniques such as:
- Cryo-electron microscopy (cryo-EM)
- NMR
- X-ray crystallography
Functional Studies
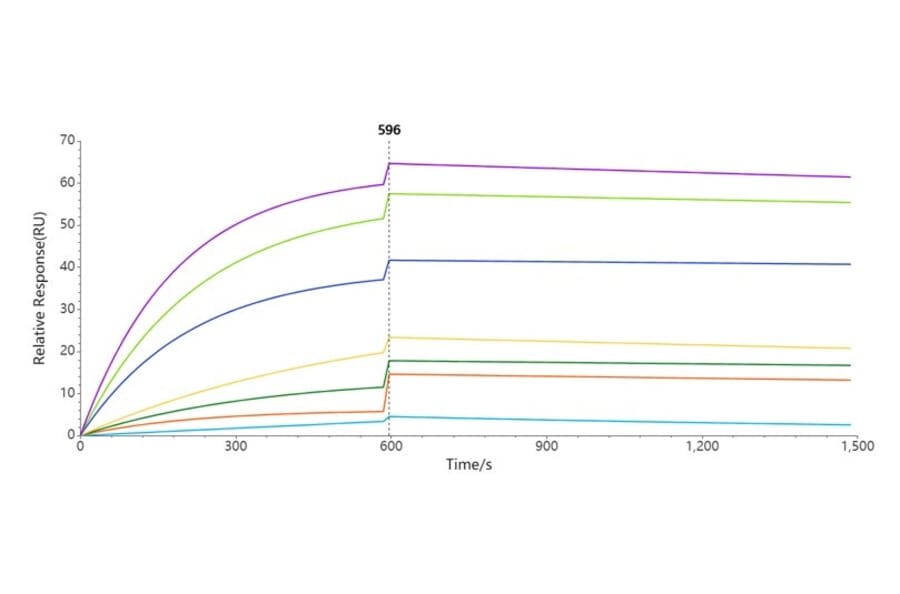
Nanodiscs can be used to study the function of membrane proteins thanks to their low viscosity and turbidity in aqueous solution, the access they provide to multiple domains of the protein, and the ability to immobilize the protein without interfering with the natural conformation of the protein.4 In single molecule studies, the protein can be immobilized to a solid surface via tags on the scaffold or lipid components, leaving the protein to interact unimpeded with ligands or binding partners.14 Nanodiscs can also act as sensors by tagging them with a split fluorophore.15
Nanodiscs are therefore suitable for a number of functional approaches, including:
- Atomic force microscopy
- Linear dichroism optical spectroscopy
- Resonance Raman spectroscopy
- Surface plasmon resonance (SPR)
- Förster resonance energy transfer (FRET)
Clinical and Therapeutic Applications
Drug Screening and Drug Discovery
As with functional studies of membrane proteins, nanodiscs are useful in drug screening approaches due to their maintenance of protein activity, accessibility to ligands (such as small molecules), and the ease of immobilizing them to solid surfaces.16 Nanodiscs can be used to generate membrane protein libraries from tissue fractions while preserving their activity, which can then be paired with high-throughput screening.17
Antibody Development
Nanodiscs are also useful when developing and characterizing antibodies that bind to membrane proteins.18,19 Antibody binding kinetics to membrane proteins can be examined by coating protein-containing nanodiscs on to a plate, and then using SPR to measure on- and off-rates of antibody binding.20 Pure membrane protein may not be feasible to obtain, purify, or keep in a stable and active conformation, all of which are required for standard antibody generation and can be solved by using nanodiscs.
Raising antibodies against membrane proteins benefits from using nanodiscs to prevent aggregation, which can block antigenic sites and lower an immune response. Membrane proteins within nanodiscs can also be used in phage- and yeast-display, a preferable alternative to using detergents.21,22
Drug Delivery
Novel methods of drug delivery are highly sought after to improve the pharmacokinetics of drugs (what happens to the drug once in the body), target drugs to specific areas, and control drug release. Based on HDLs, which are a natural transportation solution in the blood stream for lipids, proteins and RNA, nanodiscs are an ideal carrier to deliver drugs to specific sites in the body, particularly following surface modification of nanodiscs.23,24 Nanodiscs can improve the bioavailability of drugs, and load compounds that would otherwise be poorly water soluble. They have also been shown to accumulate more in tumors than other nano-particle delivery systems, in part due to their small size, stability, and long circulation half-life.24
Nanodisc delivery systems have thus far shown promise in a wide-range of therapeutic applications, including treatments for cancer, infectious diseases and neurodegenerative conditions, as well as vaccine development, diagnostic imaging, and specific targeting of drugs to the brain.23–27