A loading control effectively acts as an internal reference protein, allowing researchers to ensure that differences observed between samples are due to genuine biological variation rather than experimental error.
The protein chosen for a loading control should not vary with experimental conditions, meaning any difference seen in the levels of this protein actually reflect procedural error. Not only does this identify unintended variation, it also enables error correction by normalizing the signal of the protein of interest to the loading control signal.
See all Loading Control Antibodies Table of Contents
Loading controls vs Protein quantification
Checking that total protein concentration is consistent across samples is essential to know whether differences between samples are biological or not. Loading controls are used as a proxy for total protein content. Earlier steps in the western blotting procedure, such as a BCA, Bradford or Lowry assay, aim to keep protein content consistent prior when loading samples on to the gel. Loading controls check that loading was equal, in addition to identifying potential issues with membrane transfer and signal detection, therefore allowing the examination of biological differences between samples (Figure 1). Both loading controls and early protein quantification are recommended when performing a western blot for the most reliable and consistent results.
![Western blot witb beta-Actin loading control - Anti-Cyclin D1 Antibody [ARC0300] (A306339) - Antibodies.com](data:image/gif;base64,R0lGODlhAQABAIAAAP///wAAACH5BAEAAAAALAAAAAABAAEAAAICRAEAOw==)
Figure 1: Beta-actin loading control validating a KO experiment. Western blot using anti-Cyclin D1 antibody in WT and Cyclin D1 knockout HeLa cells. No band is observed for cyclin D1 in the right-hand lane, but total protein levels are unaffected as confirmed by the beta Actin loading control. Therefore, the knockout of Cyclin D1 has been successfully verified.
Which proteins are used as loading controls?
Loading control proteins tend to be housekeeping genes: well-understood and ubiquitously expressed proteins that are involved in the basic functioning of the cell. They are therefore unlikely to vary between cell types or to be affected by experimental treatments, though this must be verified to prevent incorrect normalization of genuine biological variation.
Considerations for choosing loading controls
- Molecular weight (MW): Loading controls should be a different MW to the protein of interest in order to distinguish their bands from each other on the western blot.
- Abundant expression: High expression means that the protein will be easily detected regardless of which cell type is under investigation.
- Saturation: the flip side of abundant expression is that the signal of proteins with extremely high expression can sometimes saturate very quickly, rendering them unsuitable for quantification or normalization. An antibody titration can be performed to optimize the antibody concentration to achieve a good signal-to-noise ratio while avoiding saturation.
- Experimental treatments: Loading controls should not be affected by the conditions of the experiment, including drug treatments and genetic knockouts. It is therefore useful to understand the biology of each loading control candidate and to study the literature to determine if an experiment will affect a classically used loading control (Figure 2).
- Comparable expression between sample types: Some experiments involve comparing different cell types, tissues or even species. A suitable loading control must have comparable expression across all sample types to allow reliable comparisons.
- Subcellular compartment: If you have sample lysates from a specific subcellular compartment, such as the nucleus, the loading control must be expressed there. Table 1 below shows common loading controls and where they are localized within the cell.
- Circadian time: Some housekeeping genes, and indeed total protein synthesis, can vary in abundance with circadian time, which will distort the interpretation of results if used as a loading control.
- Fluorescence: If performing a multiplex fluorescent western blot, the protein of interest should be detected using the brightest fluorophore, while the dimmest fluorophore should be used for the detection of the loading control, which tends to be much higher abundance.

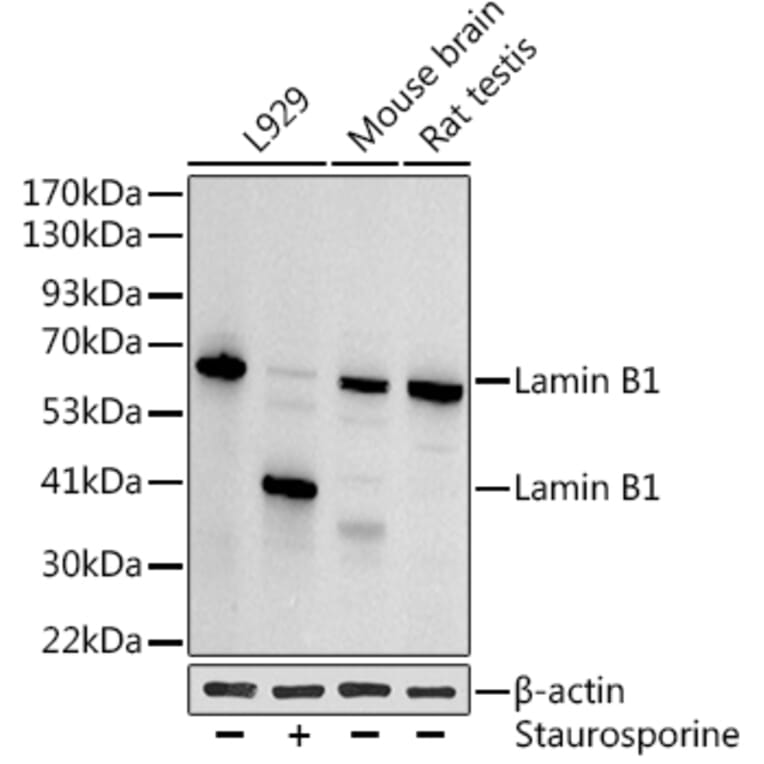
Figure 2: Loading controls can be affected by experimental conditions.Western blot using anti-Lamin B1 antibody, which can act as a nuclear loading control. However, lamin B1 is cleaved during apoptosis3, caused here by staurosporine addition, which would make it unsuitable for all experiments. Instead, beta Actin is used as a loading control.
Common Loading Controls
Table 1:Loading controls to use in western blot experiments
Loading Control Antibodies by Sample Type:
| Target | Antibody | Reactivity |
| Actin | Anti-Actin Antibody [5J11] (A85388) | Human, Horse, Cow, Porcine, Chicken, Rat, Mouse |
| Anti-Actin Antibody [HHF35] (A85719) | Human, Mouse, Rat, Canine, Feline, Rabbit, Chicken |
| beta Actin | Anti-beta Actin Antibody [BA3R] (A85272) | Chicken, Human, Mouse, Rat, Rabbit |
| Anti-beta Actin Antibody [S12-I] (A8250) | Human, Mouse, Rat |
| Anti-beta Actin Antibody [RM112] (A121404) | All Species |
| Cofilin | Anti-Cofilin Antibody (A96586) | Human, Mouse, Rat |
| Anti-Cofilin Antibody (A27001) | Human, Mouse, Rat |
| alpha Tubulin | Anti-alpha Tubulin Antibody [BA3R] (A101670) | Human, Mouse, Rat, Hamster, Xenopus, C. elegans |
| Anti-alpha Tubulin Antibody [RM113] (A121506) | All Species |
| Anti-alpha Tubulin Antibody [TU-01] (A86726) | Mouse, Bovine, Canine, Human, Porcine |
| Anti-alpha Tubulin Antibody [TU-02] (A86724) | Human, Porcine, Mouse |
| Anti-alpha Tubulin Antibody [TU-16] (A86709) | All Species |
| beta Tubulin | Anti-beta Tubulin Antibody [1B12] (A85428) | Human, Monkey, Rat, Mouse |
| Anti-beta Tubulin Antibody [4E4] (A85429) | Human, Monkey, Rat, Mouse |
| Anti-beta Tubulin Antibody [BT7R] (A85273) | Chicken, Human, Monkey, Mouse, Rat, Rabbit |
| Anti-beta Tubulin Antibody [TU-06] (A86335) | Human, Mouse, Rat, Bovine, Hamster |
| Anti-beta Tubulin Antibody [TU-13] (A86730) | Human, Porcine, Mouse, Plants |
| Target | Antibody | Reactivity |
| HDAC1 | Anti-HDAC1 Antibody (A12564) | Human, Mouse, Rat |
| Anti-HDAC1 Antibody (A84192) | Human, Mouse |
| Histone H3 | Anti-Histone H3 Antibody (A25203) | Human, Mouse, Rat |
| Anti-Histone H3 Antibody (A16702) | Human, Mouse, Rat |
| Anti-Histone H3 Antibody [RM186] (A121358) | All Vertebrates |
| Anti-Histone H3 Antibody [RM190] (A121350) | All Vertebrates |
| Lamin B1 | Anti-Lamin B1 Antibody (A82838) | Human, Mouse |
| Anti-Lamin B1 Antibody (A26974) | Human, Mouse, Rat |
| PCNA | Anti-PCNA Antibody (A83563) | Human, Mouse, Rat, Porcine |
| Anti-PCNA Antibody (A25315) | Human, Mouse, Rat |
| Anti-PCNA Antibody [PC10] (A86878) | Human, Mouse, Rat, Chicken, Drosophila |
| TBP | Anti-PCNA Antibody (A26155) | Human, Mouse, Rat |
| Anti-PCNA Antibody (A82888) | Human, Mouse, Rat |
| Target | Antibody | Reactivity |
| Cyclophilin B | Anti-Cyclophilin B Antibody [Pk2E2AT] (A58461) | Human |
| GAPDH | Anti-GAPDH Antibody (A83722) | Human, Mouse, Rat |
| Anti-GAPDH Antibody (A85377) | Human, Horse, Cow, Porcine, Chicken, Rat, Mouse |
| Anti-GAPDH Antibody [1D4] (A85382) | Human, Horse, Cow, Porcine, Chicken, Rat, Mouse |
| Anti-GAPDH Antibody [GA1R] (A85271) | Chicken, Hamster, Human, Mouse, Rat, Rabbit, Sf9 Insect, BL-21 Bacteria, S. cerevisiae |
| Anti-GAPDH Antibody [RM114] (A121396) | Human, Monkey |
| Vinculin | Anti-Vinculin Antibody (A94871) | Human, Mouse, Rat |

![Western blot witb beta-Actin loading control - Anti-Cyclin D1 Antibody [ARC0300] (A306339) - Antibodies.com](https://cdn.antibodies.com/image/catalog/306/A306339_1.jpg?profile=product_top)