+1 (314) 370-6046 or
Contact Us - Argentina
- Australia
- Austria
- Bahrain
- Belgium
- Brazil
- Bulgaria
- Cameroon
- Canada
- Chile
- China
- Colombia
- Croatia
- Cyprus
- Czech Republic
- Denmark
- Ecuador
- Egypt
- Estonia
- Finland
- France
- Germany
- Greece
- Hong Kong
- Hungary
- Iceland
- India
- Indonesia
- Iran
- Ireland
- Israel
- Italy
- Japan
- Kazakhstan
- Kuwait
- Latvia
- Lithuania
- Luxembourg
- Macedonia
- Malaysia
- Malta
- Mexico
- Monaco
- Morocco
- Netherlands
- New Zealand
- Nigeria
- Norway
- Peru
- Philippines
- Poland
- Portugal
- Qatar
- Romania
- Russia
- Saudi Arabia
- Serbia
- Singapore
- Slovakia
- Slovenia
- South Africa
- South Korea
- Spain
- Sri Lanka
- Sweden
- Switzerland
- Taiwan
- Thailand
- Turkey
- Ukraine
- UAE
- United Kingdom
- United States
- Venezuela
- Vietnam

![Flow Cytometry - Anti-STAT1 Antibody [SM1] (A85869) - Antibodies.com](https://cdn.antibodies.com/image/catalog/85/A85870_279.jpg?profile=product_top)
![Flow Cytometry - Anti-STAT1 Antibody [SM1] (A85869) - Antibodies.com](https://cdn.antibodies.com/image/catalog/85/A85870_279.jpg?profile=product_top_thumb)
![Flow Cytometry - Anti-STAT1 Antibody [SM1] (A85869) - Antibodies.com](https://cdn.antibodies.com/image/catalog/85/A85870_279.jpg?profile=product_image)

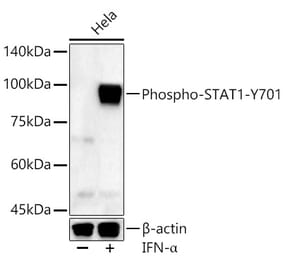
![Western Blot - Anti-STAT1 (phospho Tyr701) Antibody [ARC0049] (A308896) - Antibodies.com](https://cdn.antibodies.com/image/catalog/308/A308896_1.jpg?profile=product_alternative)
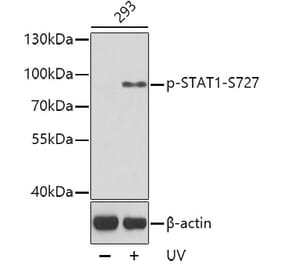
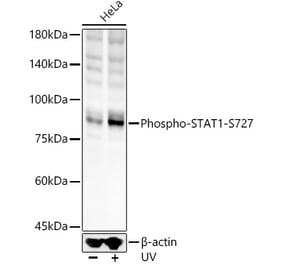
![Western Blot - Anti-STAT1 (phospho Ser727) Antibody [ARC1544] (A308199) - Antibodies.com](https://cdn.antibodies.com/image/catalog/308/A308199_1.jpg?profile=product_alternative)
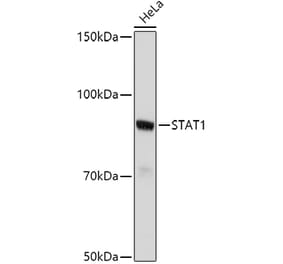
![Western Blot - Anti-STAT1 Antibody [ARC0042] (A307829) - Antibodies.com](https://cdn.antibodies.com/image/catalog/307/A307829_1.jpg?profile=product_alternative)