Ryan Hamnett, PhD | 6th April 2024
ELISAs use antibodies to detect and quantify the amount of a target antigen in a liquid sample. Controls are essential for confirming the accuracy, reliability and sensitivity of ELISAs, and can assist with troubleshooting in case of spurious results.
| Control | Type of control | ELISA format | Description | Purpose | Notes |
|---|---|---|---|---|---|
| Blank control | Negative | All | Empty well or well filled only with ELISA buffer | Determines contribution of plate plastic and buffer to absorbance measurements | Often omitted in favor of wavelength correction and S0 or NSB controls (see below) |
| S0 (Zero standard) control | Negative | Direct, indirect, sandwich | All steps of ELISA are performed in absence of any analyte from standard or sample | Identifies maximal background due to non-specific binding of the detection antibody | Absorbance value can be used to correct measurements for samples, or as the lowest point on standard curve |
| Non-specific binding control | Negative | Competitive using enzyme-conjugated analyte | Competitive ELISA is run in the absence of sample or standards | Similar to S0, this identifies background due to non-specific binding of an enzyme-conjugated analyte | “NSB” is sometimes also used as a general term to refer to S0 controls and secondary antibody controls |
| Secondary antibody control | Negative | Indirect, indirect sandwich | Indirect or indirect sandwich ELISA is run in the absence of primary or detection antibody, respectively | Identifies background caused only by non-specific binding of the labeled secondary antibody | Sometimes grouped in as an NSB control. Useful in troubleshooting |
| Wavelength control | Negative | All | Absorbance is measured at a different wavelength that the colored compound does not absorb at | Identifies non-specific absorbance, usually contributed by the plate plastic, scratches | Subtract OD at different wavelength (e.g. 570 nm) to get true OD at specific wavelength (e.g. 450 nm). Usually, this subtraction is automatically carried out by a plate reader |
| Negative matrix control | Negative | All, particularly those with a complex sample matrix | ELISA is run using a sample matrix guaranteed not to contain any analyte | Determines contribution of analyte-independent sample matrix (e.g. interfering factors) to final absorbance | Theoretically useful, but in practice it can be difficult to get an equivalent sample guaranteed not to contain any analyte |
| Standards | Positive | All | As described in our ELISA Overview guide, standards are known concentrations of purified antigen | Tests assay’s functionality, and enables quantitative assessment of samples | Essential to run in all quantitative ELISAs |
| B0 control | Positive | Competitive | Procedurally the same as S0. All steps of competitive ELISA are performed in absence of any analyte from standard or sample | Unlike S0 in standard ELISAs, which is a negative control, B0 will result in maximum color development in competitive ELISAs. Checks for full functionality of all assay components | Can be used as a reference against which to compare test values, as a percentage, e.g. %B or B/B0, therefore recommended for all competitive ELISAs |
| Total activity (TA) control | Positive | All | Small amount of enzyme-conjugated protein and substrate are incubated together during color development, then stopped with stop buffer. No sample or primary antibody (if relevant) are needed | Ensures enzymatic activity | Qualitative control only, not used in any quantification |
| Positive matrix control | Positive | All, particularly those with a complex sample matrix | Same matrix as sample, but known to contain endogenous analyte | Confirms assay is working as expected. If quantity of analyte is known, matrix interference can be determined by comparing to known standards | Spiked matrix control is often preferred because it is easier know analyte concentration |
| Spiked matrix control | Positive | All, particularly those with a complex sample matrix | Sample matrix containing little or no analyte is spiked with a known quantity of purified antigen | Confirms assay is working as expected, and identifies matrix interference when compared to standards in standard diluent | Ideally spike recovery will be 100% - greater than 20% deviation (80-120%) suggests matrix interference |
| Endogenous protein control | Positive | Only during ELISA development alongside recombinant standards | Run ELISA using known quantity of endogenous protein alongside recombinant standards | Confirms that recombinant standards are behaving in the same way as endogenous protein, e.g. epitope is accessible | Not necessary during most ELISA runs |
| Linearity of dilution control | Optimization | All | Serially dilute a sample and perform a full ELISA | For identifying matrix interference. Once values show a linear relationship with the dilution factor, matrix interference is negligible | Samples should only be run if they have been diluted to the appropriate range, once established. Linearity of dilution should be validated for every type of assay, sample matrix and analyte. Can be generated from spiked matrix samples |
Table 1:Key controls to run alongside an ELISA experiment.
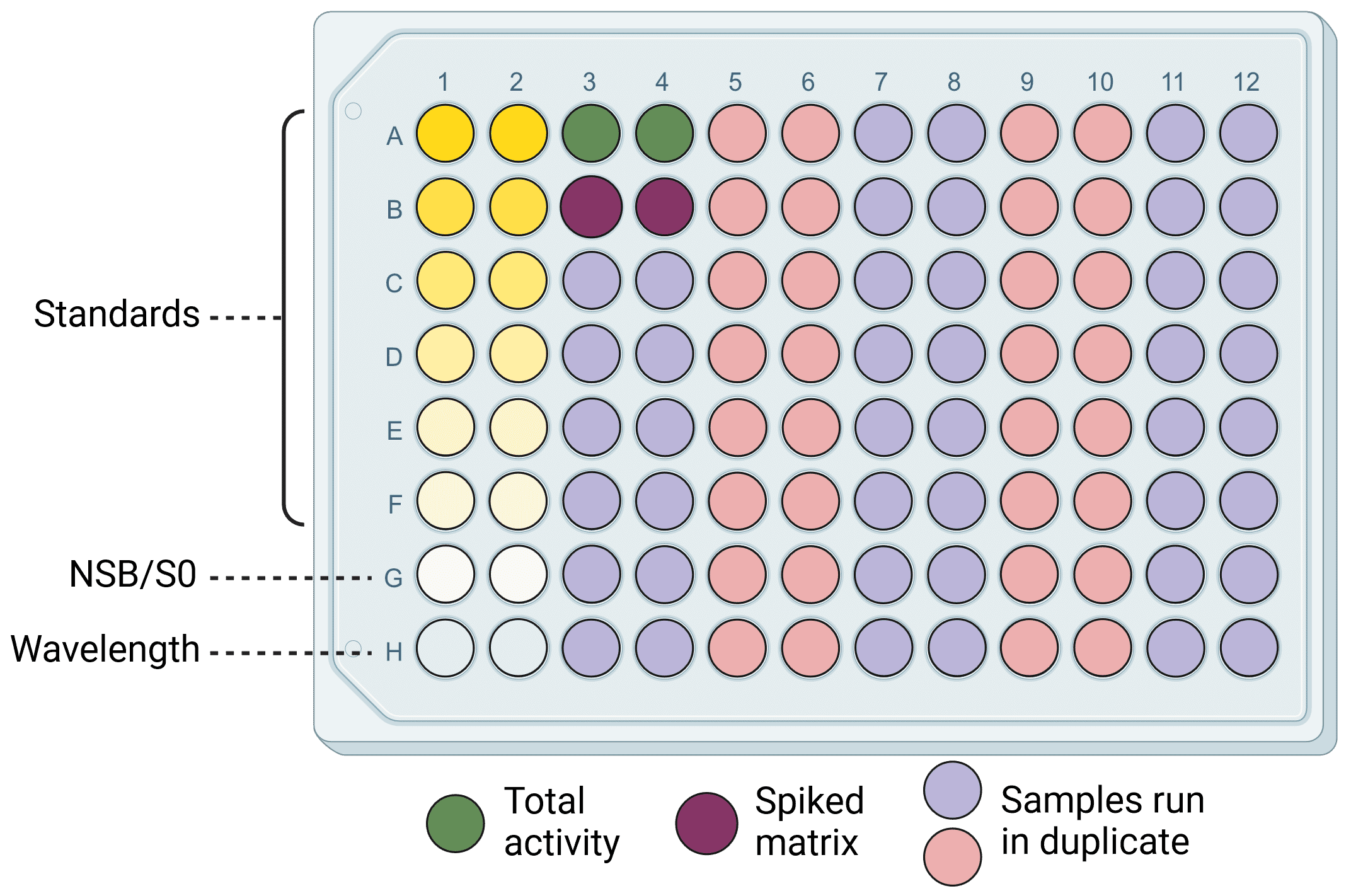
Figure 3: An example ELISA plate layout. Wells include standard dilutions to generate a standard curve, negative (NSB/SO and wavelength) and positive (total activity and spiked matrix) controls, and 38 samples. Everything is run in duplicate for greater accuracy and reliability. Diagram made with BioRender.