Clinical Performance:
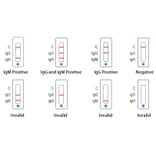
The in vitro diagnostic reagents used in the test are compared with the clinical diagnostic criteria of new coronavirus pneumonia to verify the clinical performance of this product. The enrolled cases were suspected cases of new coronavirus infection, a total of 1,585 cases, including 421 confirmed cases and 1,164 excluded cases. A comparative study was performed using in vitro diagnostic reagents for testing and the clinical diagnostic criteria of new coronavirus pneumonia. The test results show that the product has a clinical sensitivity of 98.81% (95% CI: 97.25%, 99.61%) and a clinical specificity of 98.02% (95% CI: 97.05%, 98.74%). In addition, 203 subjects received homologous serum / plasma and whole blood specimens (125 of which were positive and 78 were negative) for comparative tests. The results show that the product is based on the serum / plasma test results, and the consistency rate of the whole blood test results is 96.85% (95% CI: 95.87% to 97.60%). After preliminary evaluation, it is basically confirmed that the clinical performance of COVID-19 Rapid Test Kit IgG + IgM (Colloidal Gold) can meet the emergency use requirements of the epidemic.
Interfering Substances:
1. When the bilirubin concentration is = 250 µmol/L, the hemoglobin content is = 9 g/L, the triglyceride content is = 15 mmol/L, the rheumatoid factor content is = 80 IU/mL, and the anti-nuclear antibody (ANA) titer is = 1: 240, anti-mitochondrial antibody (AMA) = 80 U/mL, and mouse IgG content = 1000 µg/mL, it will not interfere with the detection results of this product.
2. Histamine hydrochloride, alpha-interferon, zanamivir, ribavirin, oseltamivir, peramivir, lopinavir, ritonavir, abidol, levofloxacin, azithromycin, Ceftriaxone, meropenem, and tobramycin have been validated to have no effect on the test results of this product.
Cross Reactivity: This COVID-19 Rapid Test Kit IgG + IgM (Colloidal Gold) does not cross react with positive samples of parainfluenza virus antibody, influenza A virus antibody, influenza B virus antibody, chlamydia pneumoniae antibody, mycoplasma pneumoniae antibody, adenovirus antibody, respiratory syncytial virus antibody, hepatitis B surface antibody, type C Hepatitis virus antibody, treponema pallidum antibody, human immunodeficiency virus antibody, EB virus antibody, measles virus antibody, cytomegalovirus antibody, enterovirus 71 antibody, mumps virus antibody, chicken pox-zoster virus, and HKU1 virus antibodies, OC43 virus antibodies, NL63 virus antibodies, and 229E virus antibodies (the other common coronavirus strains).
Further Information:
If you require any further information, then please do not hesitate to contact us by email, telephone, or live chat. Our technical support email address is technical@antibodies.com.