Product Validation
As a company of scientists, we understand how important it is to work with products that have been validated for specificity, selectivity, activity, stability, and performance.
We work hard to ensure that every product is validated in multiple research applications and with samples derived from multiple species, and we share this data on our website so that you can have confidence in our reagents. Not only does our validation process test our products with a variety of techniques, including western blot, ICC/IF, ELISA, flow cytometry, immunoprecipitation and chromatin immunoprecipitation, many are also subjected to multiple levels of specificity validation, including knockout validation and protein arrays.
Table of Contents
Knockout validation confirms that specific signal is lost when an antibody is tested against a sample that has had the target protein genetically knocked out. We have incorporated knockout validation, using CRISPR/Cas9, as part of our standard antibody validation process, as indicated by the Knockout Validated seal on selected products.
An increasing number of our antibodies are now tested for specificity and selectivity against 19,000+ full-length human proteins on human genome-wide protein arrays. These arrays compare the binding of an antibody to its target protein and the binding to off-target proteins, providing a complete overview of the specificity and selectivity of an antibody. With our protein array-validated antibodies, researchers can be confident they are using precision tools.
Figure 3: Analysis of a protein array containing more than 19,000 full-length human proteins using Anti-beta III Spectrin Antibody [SPTBN2/1584] (A253226). Z-Score and S- Score: The Z-score represents the strength of a signal that a monoclonal antibody (MAb) (in combination with a fluorescently-tagged anti-IgG secondary antibody) produces when binding to a particular protein on the HuProt™ array. Z-scores are described in units of standard deviations (SD's) above the mean value of all signals generated on that array. If the results for the targets on the HuProt™ array are arranged in descending order of the Z-score, the S-score is the difference (also in units of SD's) between the Z-scores. S-score therefore represents the relative target specificity of a MAb to its intended target; a MAb is considered to be specific to its intended target if the MAb has an S-score of at least 2.5. For example, if a MAb binds to protein X with a Z-score of 43 and to protein Y with a Z-score of 14, then the S-score for the binding of that MAb to protein X is equal to 29.
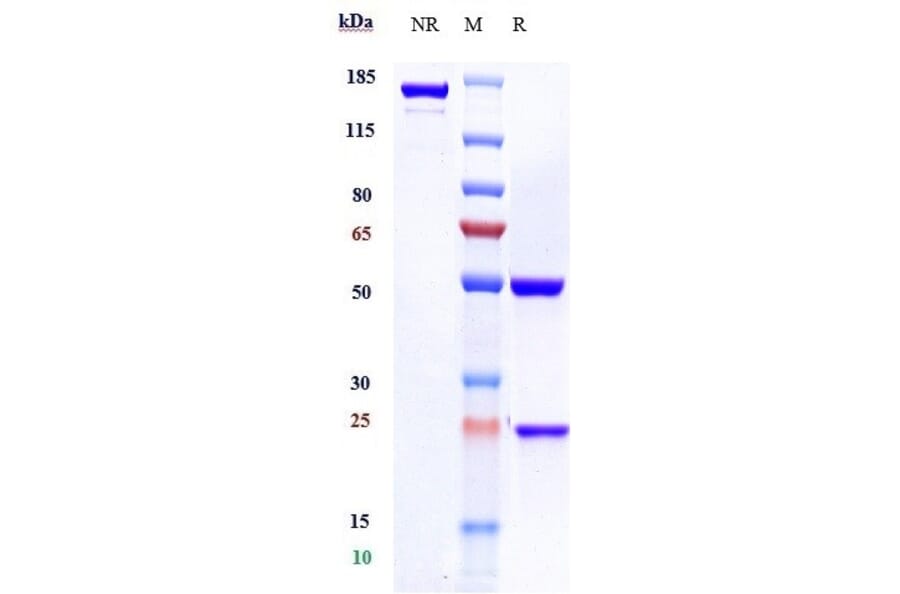
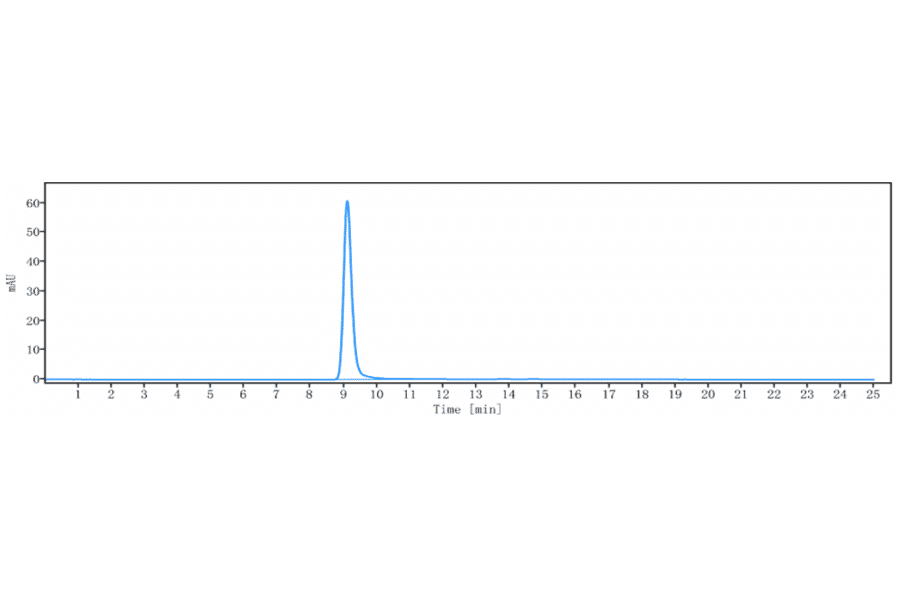
It is essential that a product is as pure as possible to be confident in assigning observed activity or signal to the product itself, rather than impurities such as cofactors or aggregates. SDS-PAGE has traditionally been used to assess purity, but size exclusion high-performance liquid chromatography (SEC-HPLC) has become the gold standard technique to determine protein purity and aggregation and ensure batch consistency. SEC-HPLC is a high-throughput and high-sensitivity biophysical method that, compared to SDS-PAGE, employs mild conditions to separate molecules based on their size.
We have recently launched a collection of SEC-HPLC validated antibodies, which includes over 900 biosimilar antibodies.
Functional and In vivo Validation
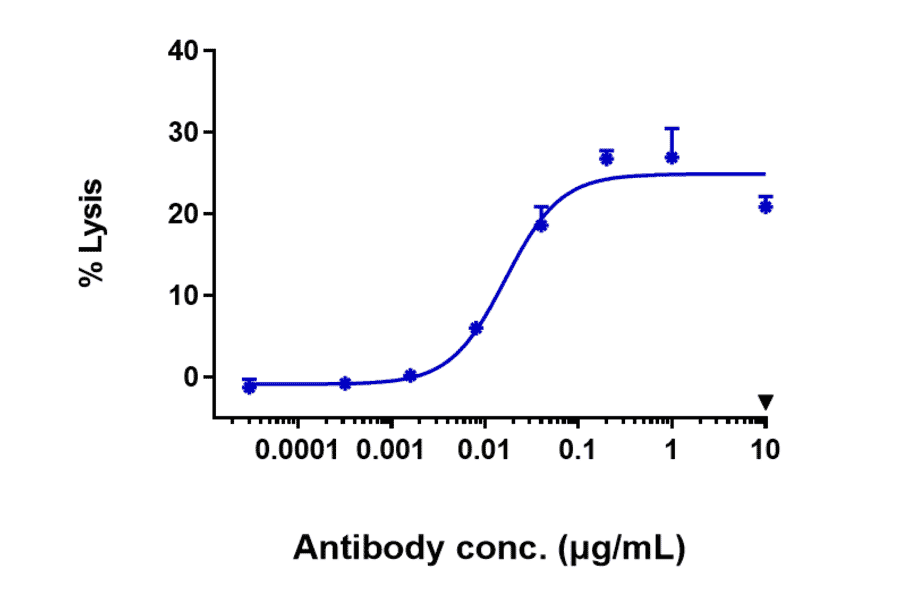
Our biosimilar antibody catalog also benefits from functional and in vivo testing to confirm that these tools mimic the activity expected of therapeutic antibodies. As appropriate, such testing includes:
- Extensive bioactivity testing – for example FACS or ELISA across a number of antibody concentrations
- Antibody dependent cellular cytotoxicity (ADCC)
- Antibody internalization by endocytosis
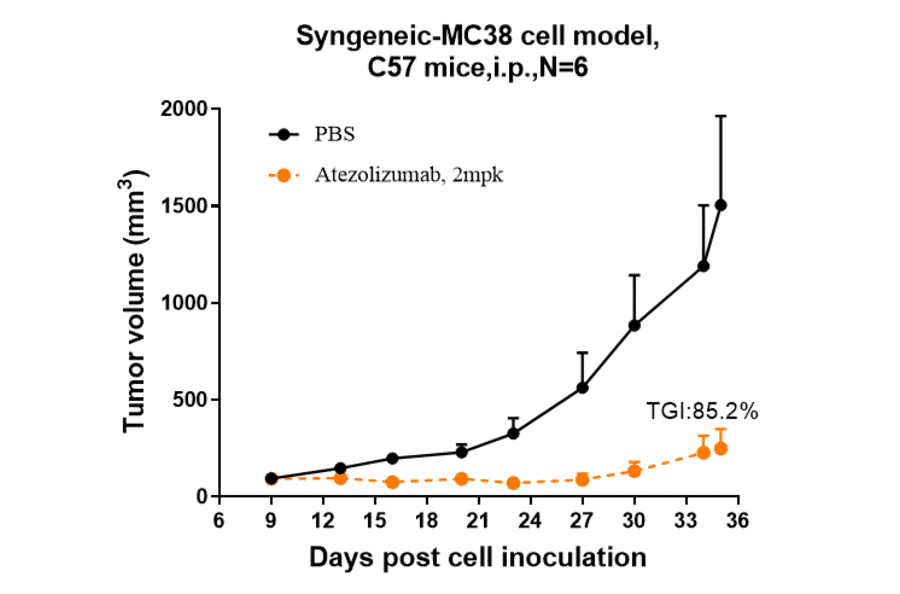
- Relevant in vivo assessments, such as tumor growth suppression
Species-specific Validation
The reactivity of an antibody with homologues from different species can be a desired feature, making the product more versatile, or an unwanted side-effect. We share all of our specificity data on our website enabling you to make informed purchasing decisions.

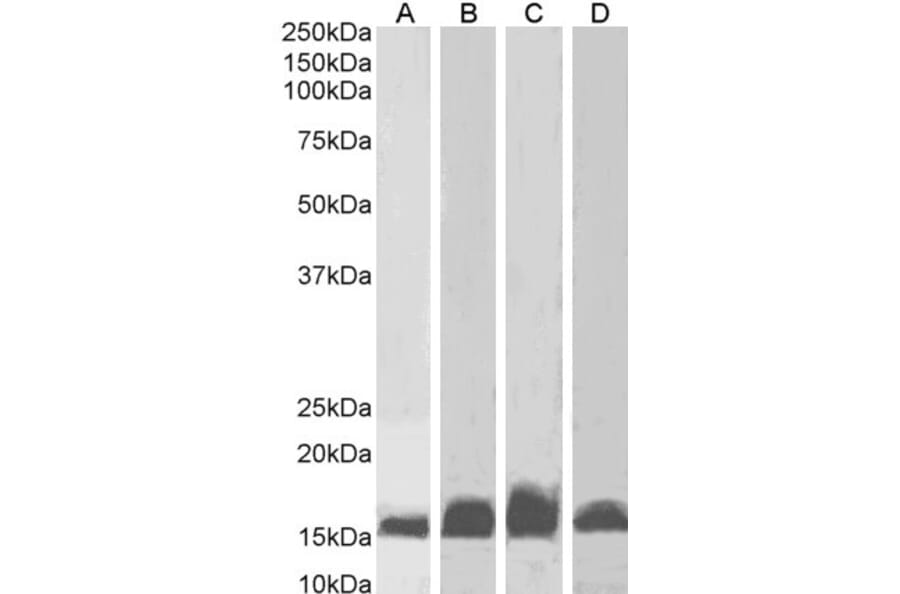
Figure 8: Anti-AIF1 Antibody (A82670) (1µg/ml) staining of Human Frontal Cortex (A), Rat Brain (B), Mouse (C) Brain and Pig Brain (D) lysates (35µg protein in RIPA buffer). Detected by chemiluminescence.

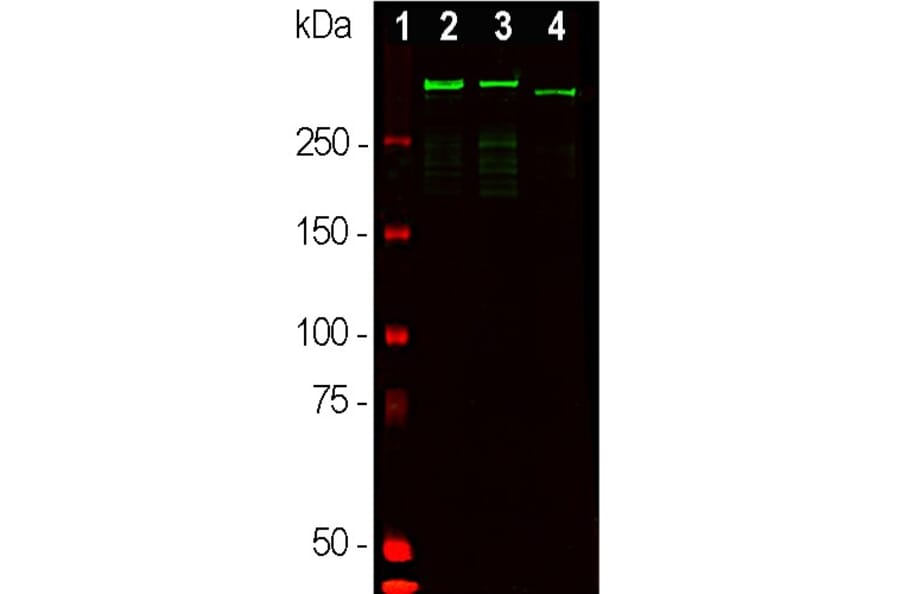
Figure 9: Western blot analysis of whole brain tissue lysates using Anti-MAP2 Antibody (A85363) (1:50,000, green): [1] protein standard (red), [2] adult rat brain, [3] embryonic E20 rat brain, [4] adult mouse brain.
Future Methods – Biophysical Validation
We are constantly striving to improve and expand our portfolio of life science reagents, including recombinant antibodies, transmembrane proteins, and aptamers. New products require new forms of validation, while ever more precise tools for validation become available to ensure the highest quality products reach our customers.
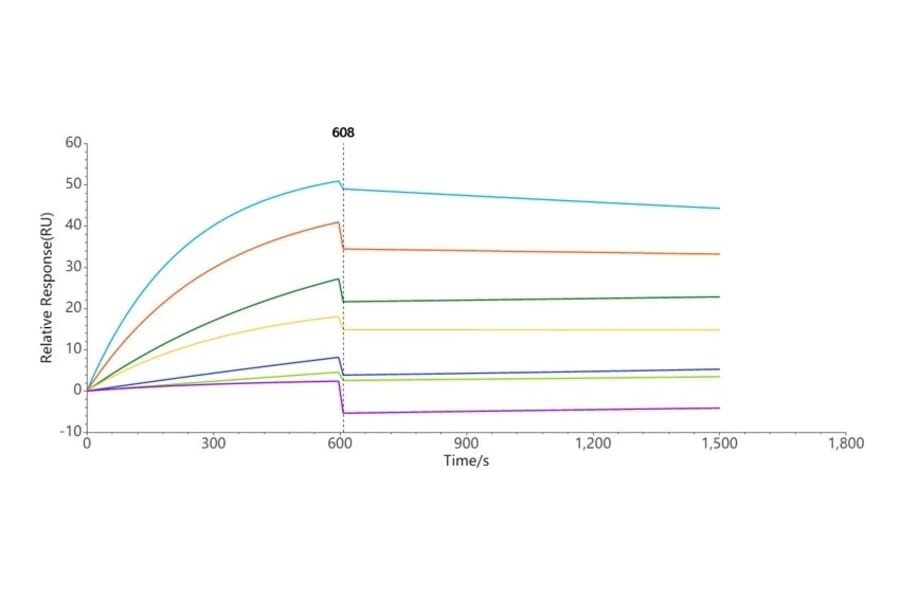
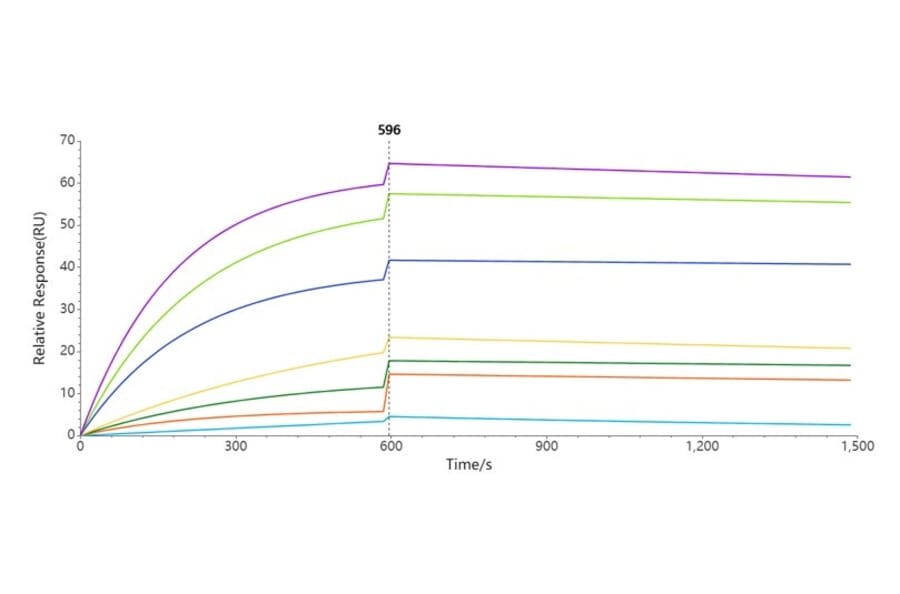
Biophysical methods of validation provide detailed insights into the structure, stability and activity of biologics, guaranteeing the conformation and function of proteins are as expected. One advanced analytical approach that we are beginning to introduce for our nanodisc transmembrane proteins is surface plasmon resonance (SPR), which is a non-destructive optical method for determining binding kinetics of proteins to analytes.
Product validation is at the heart of our business, providing the highest caliber products so that our customers can have confidence in their results. Multiple levels of validation, including knockout studies, protein arrays and functional tests, allow us to guarantee the quality of our reagents.
We encourage all our customers to submit product reviews and share what works and what doesn’t. All reviews are published online, allowing scientists to learn about product performance, new applications, optimal dilutions, and to see images of our products at work.

Figure 12: Immunofluorescent analysis of primary cultured astrocytes using Anti-GFAP Antibody (A85419) (green) and DAPI nuclear DNA staining (blue). Data submitted in a customer product review.


![Western Blot - Anti-Cyclin D1 Antibody [ARC0300] (A306339) - Antibodies.com](https://cdn.antibodies.com/image/catalog/306/A306339_1.jpg?profile=product_image)
![Protein Array - Anti-beta III Spectrin Antibody [SPTBN2/1584] - BSA and Azide free (A253226) - Antibodies.com](https://cdn.antibodies.com/image/catalog/Antibodies/A250/A250046_9.jpg)