Principle of Assay
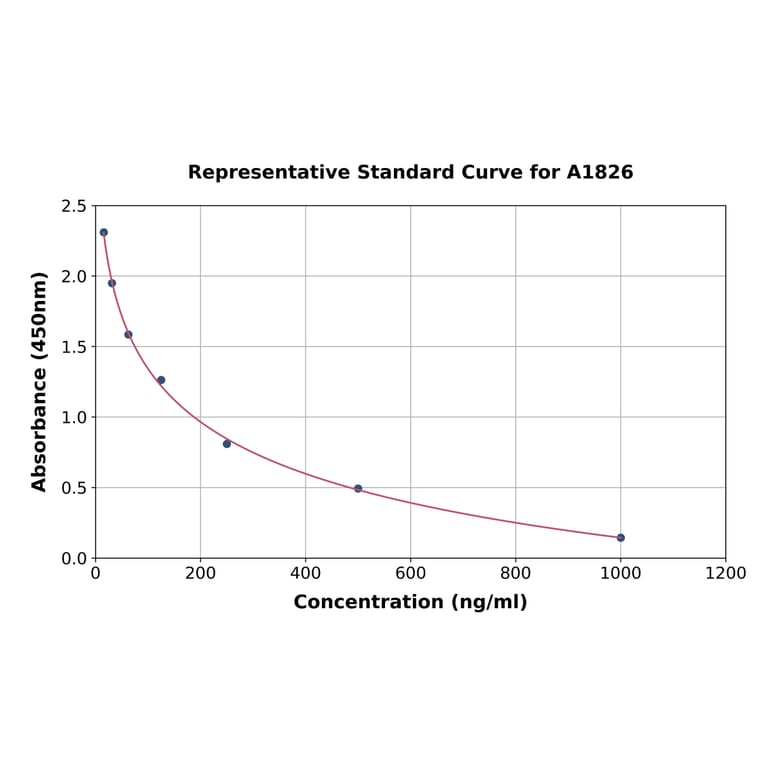
5-Hydroxytryptamine ELISA Kit (A1826) employs the competitive enzyme immunoassay technique for the quantitative measurement of 5-Hydroxytryptamine in serum, plasma or other biological fluids. The 96-well microtiter plate has been pre-coated with 5-Hydroxytryptamine antigen. During the incubation, 5-Hydroxytryptamine present in the samples or standards competes with the fixed amount of immobilized 5-Hydroxytryptamine for binding sites on the Biotinylated Anti-5-Hydroxytryptamine Antibody. The more 5-Hydroxytryptamine present in a sample or standard, the less Biotinylated Anti-5-Hydroxytryptamine Antibody that binds to the plate. Following incubation, unbound Biotinylated Anti-5-Hydroxytryptamine Antibody is removed by washing, and an HRP-Avidin conjugate is added to the wells and the microtiter plate is incubated. Following incubation and washing, TMB substrate solution is then used to visualize the HRP enzymatic reaction by catalysis to produce a blue-coloured product that changes to yellow after addition of acidic stop solution. The density of yellow is inversely proportional to the amount of 5-Hydroxytryptamine present in each sample or standard. The concentration of 5-Hydroxytryptamine can then be calculated by reading the O.D. absorbance at 450nm in a microplate reader and referring to the standard curve.